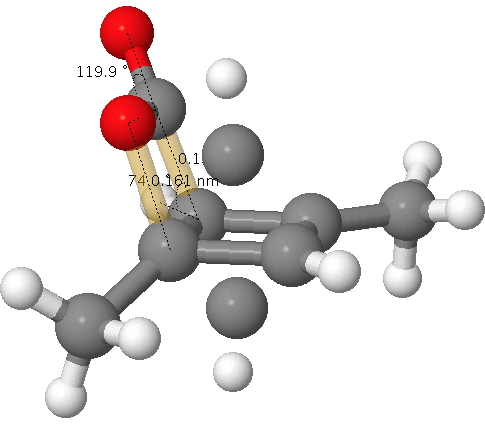
A quartet of articles has recently appeared on the topic of cyclobutadiene.,,,. You will find a great deal discussed there, but I can boil it down to this essence.

A quartet of articles has recently appeared on the topic of cyclobutadiene.,,,. You will find a great deal discussed there, but I can boil it down to this essence.
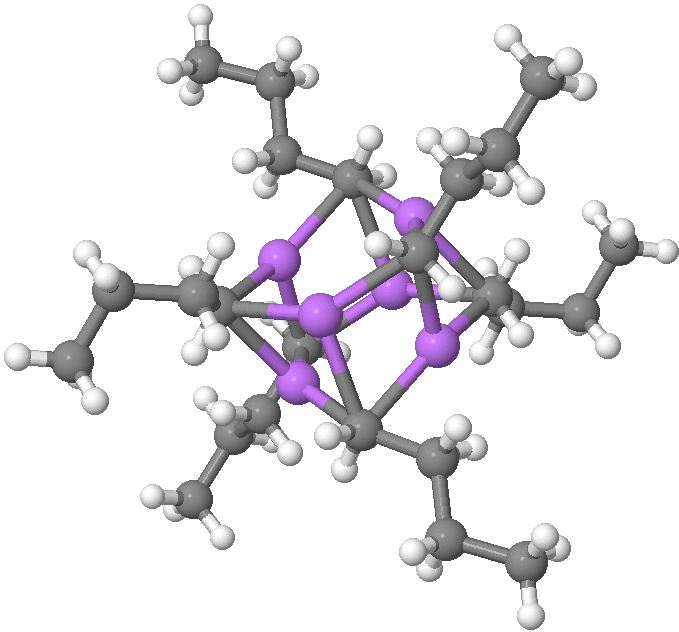
n-Butyl lithium is hexameric in the solid state and in cyclohexane solutions. Why? Here I try to find out some of its secrets.
Functionalisation of a (hetero)aromatic ring by selectively (directedly) removing protons using the metal lithium is a relative mechanistic newcomer, compared to the pantheon of knowledge on aromatic electrophilic substitution. Investigating the mechanism using quantum calculations poses some interesting challenges, ones I have not previously discussed on this blog.

William Henry Perkin is a local chemical hero of mine. The factory where he founded the British (nay, the World) fine organic chemicals industry is in Greenford, just up the road from where we live. The factory used to be close to the Black Horse pub (see below) on the banks of the grand union canal.
I mentioned in the last post that one can try to predict the outcome of electrophilic aromatic substitution by approximating the properties of the transition state from those of either the reactant or the (presumed Wheland) intermediate by invoking Hammond’s postulate. A third option is readily available nowadays; calculate the transition state directly.
The electrophilic substitution of indoles is a staple of any course on organic chemistry.
Infra-red spectroscopy of molecules was introduced 110 years ago by Coblentz as the first functional group spectroscopic method (” The structure of the compound has a great influence on the absorption spectra.
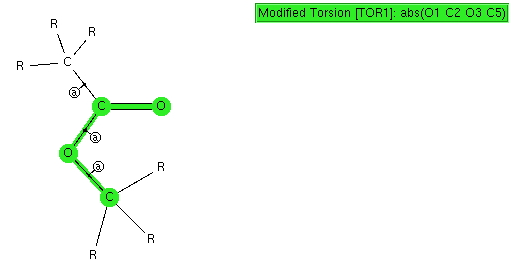
I conclude my exploration of conformational preferences by taking a look at esters. As before, I start with a search definition, the ester being restricted to one bearing only sp3 carbon centers.
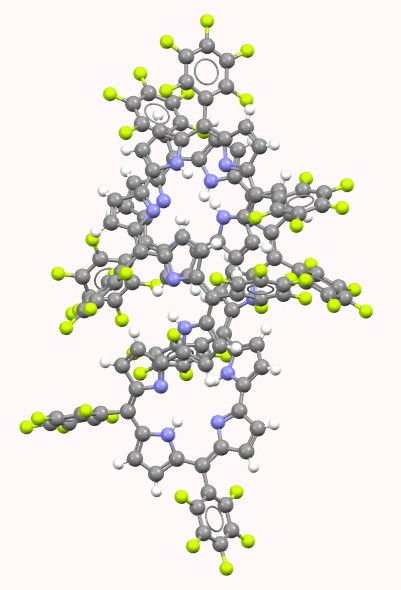
My last comment as appended to the previous post promised to analyse two so-called extended porphyrins for their topological descriptors.
An extensive discussion developed regarding my post on a fascinating helical [144]-annulene.
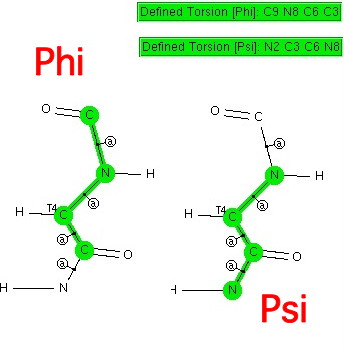
This is really just a postscript to the previous post.