This is another of those textbook reactions, involving reaction of a carbonyl compound with a phosphonium ylid to form an alkene and a phosphine oxide. The reaction continues to be frequently used, in part because it can be highly stereospecific.
The Curtius reaction is represented in most chemistry texts and notes as following path (a) below. It is one of a general class of thermally induced rearrangement which might be described as elimination/migration (in a sense similar to this ring contraction migration/elimination), in this case implicating a nitrene intermediate if the two steps occur consecutively.

Two years ago, I discussed how curly arrow pushing is taught, presenting four different ways of showing the arrows. One of the comments posted to that blog suggested that all of the schemes shown below were deficient in one aspect.
organic chemistry. It does not look like much, but this small little molecule brought us ferrocene, fluxional NMR, aromatic anions and valley-ridge inflexion points. You might not have heard of this last one, but in fact I mentioned the phenomenon in my post on nitrosobenzene. As for being at a crossroads, more like a Y-junction.
Recollect, Robinson was trying to explain why the nitroso group appears to be an o/p director of aromatic electrophilic substitution. Using σ/π orthogonality, I suggested that the (first ever) curly arrows as he drew them could not be the complete story, and that a transition state analysis would be needed. Here it is.

A month or so ago at a workshop I was attending, a speaker included in his introductory slide a QR (Quick Response) Code. It is a feature of most digital eco-systems that there is probably already “an app for it”. So I thought I would jump on the band wagon by coding an InChI string.
I blogged about this two years ago and thought a brief update might be in order now. To support the discussions here, I often perform calculations, and most of these are then deposited into a DSpace digital repository, along with metadata.
The discussion appended to the post on curly arrows is continued here.
Little did I imagine, when I discovered the original example of using curly arrows to express mechanism, that the molecule described there might be rather too anarchic to use in my introductory tutorials on organic chemistry. Why? It simply breaks the (it has to be said to some extent informal) rules!
Twenty years are acknowledged to be a long time in Internet/Web terms.
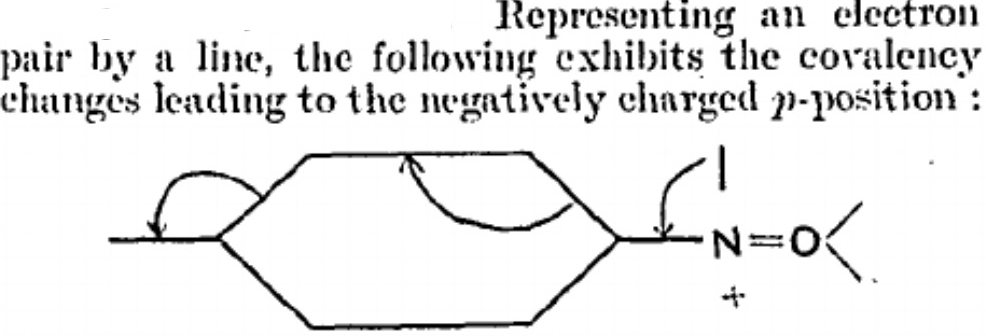
I was first taught curly arrow pushing in 1968, and have myself taught it to many a generation of student since. But the other day, I learnt something new.