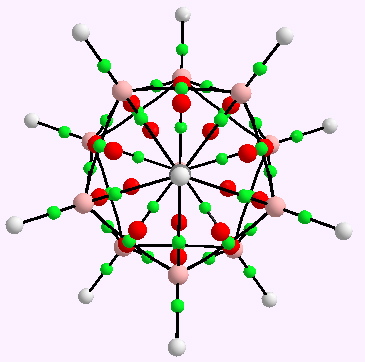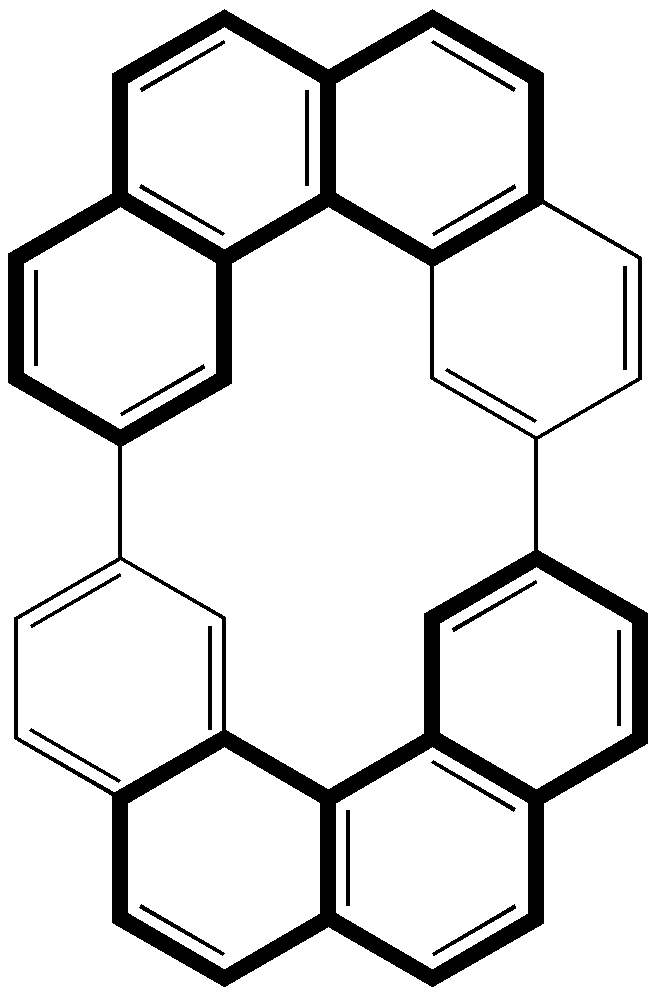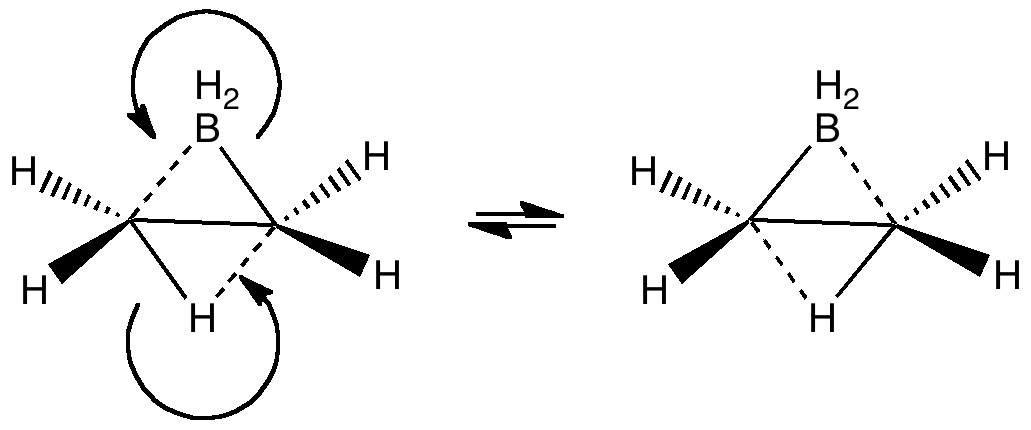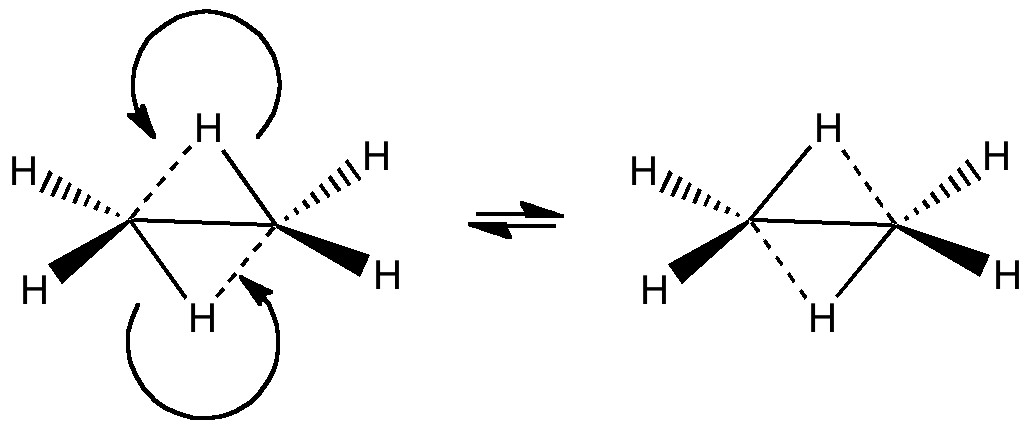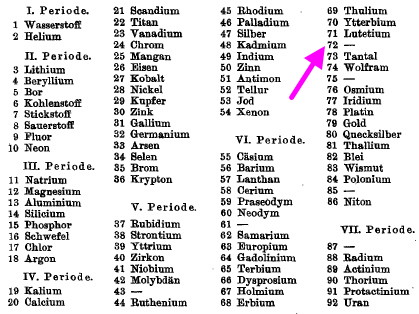
Computers and I go back a while (44 years to be precise), and it struck me (with some horror) that I have been around them for ~62% of the modern computing era (Babbage notwithstanding, ~1940 is normally taken as the start of the modern computing era). So indulge me whilst I record this perspective from […]