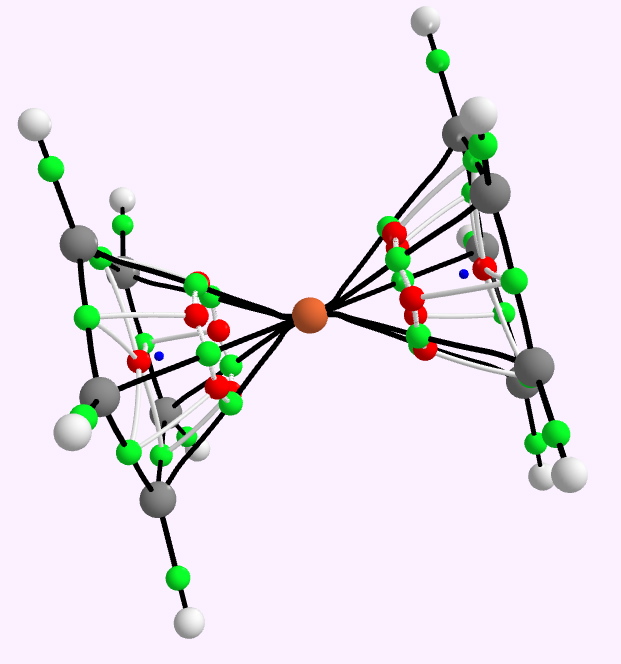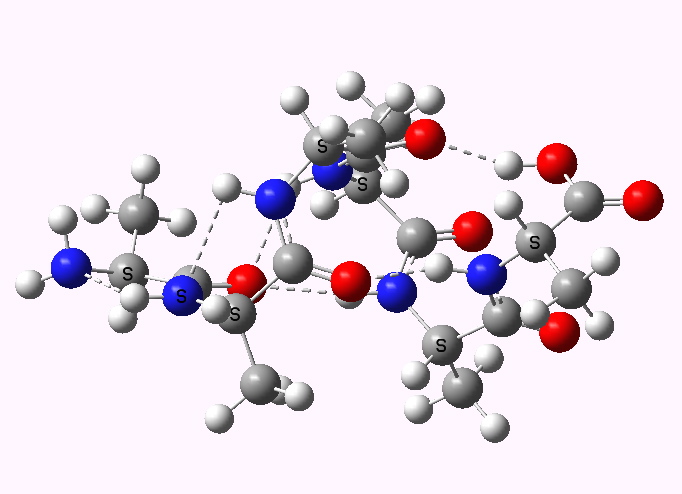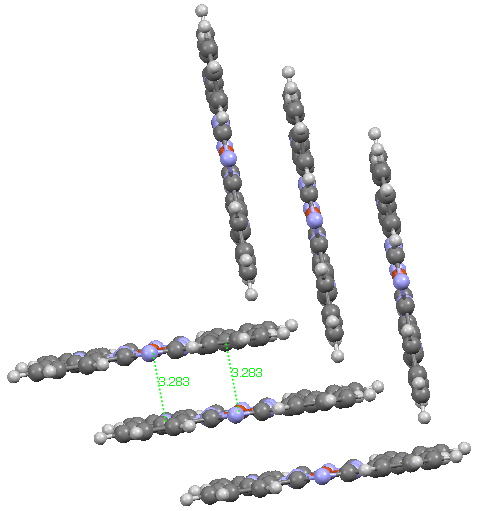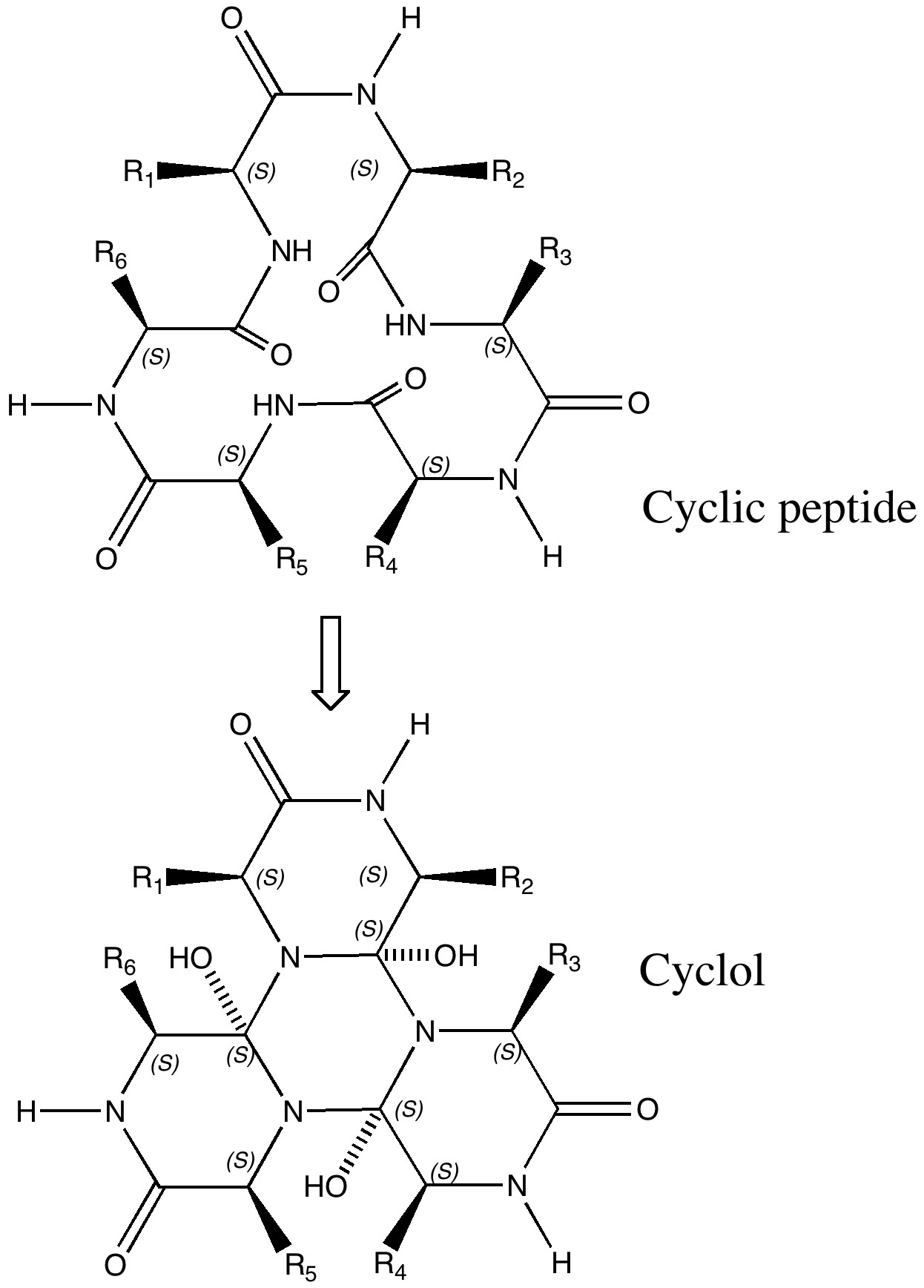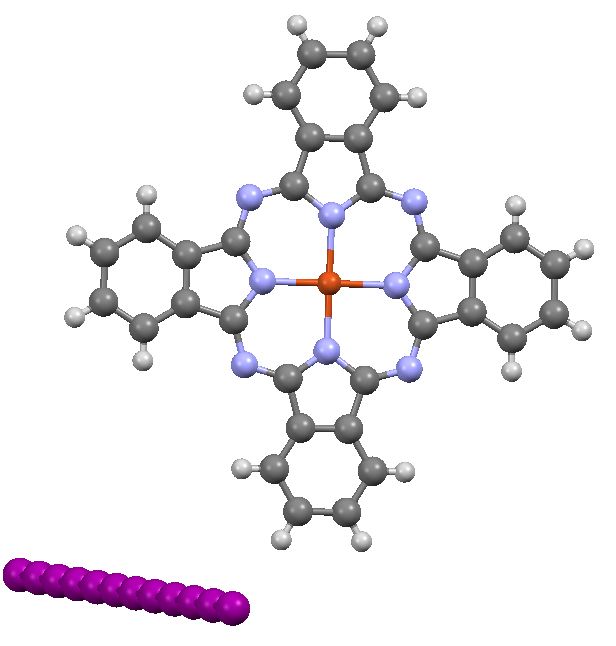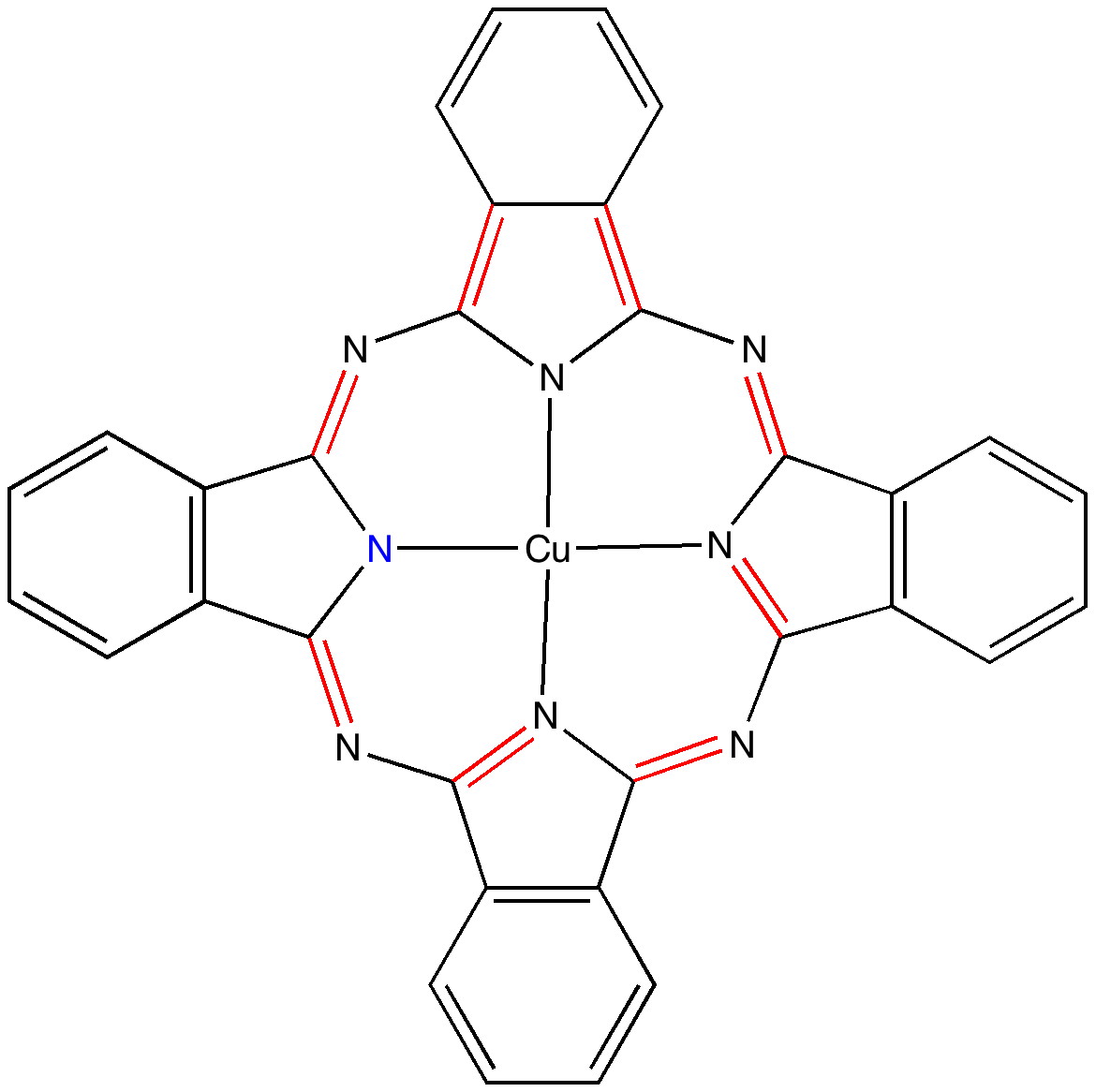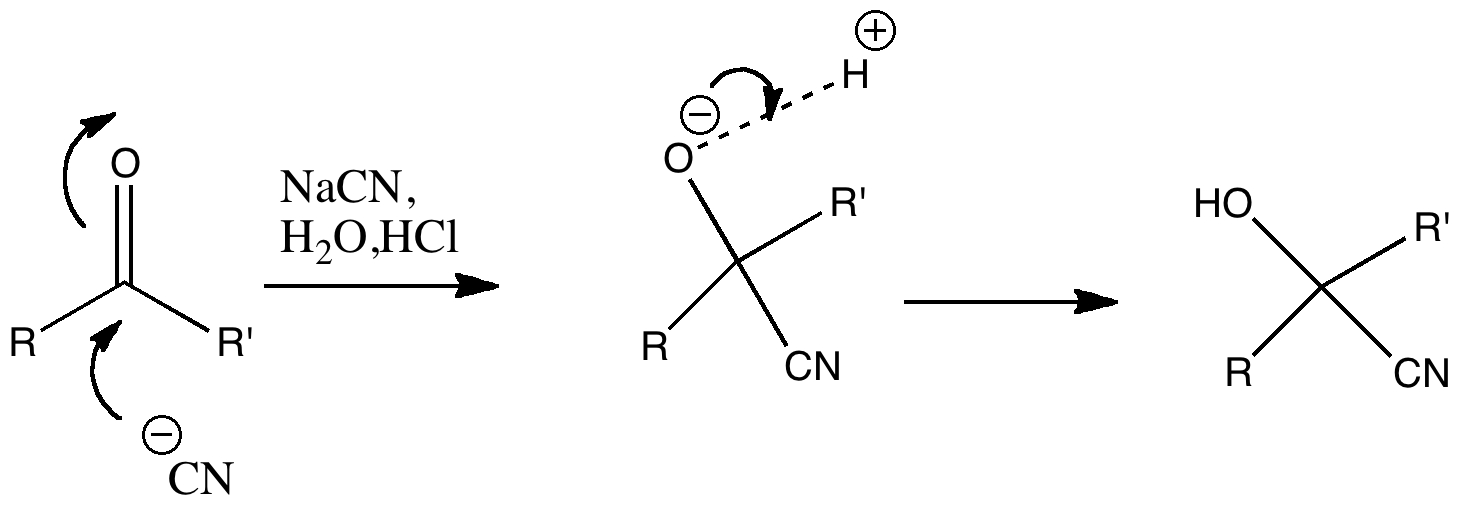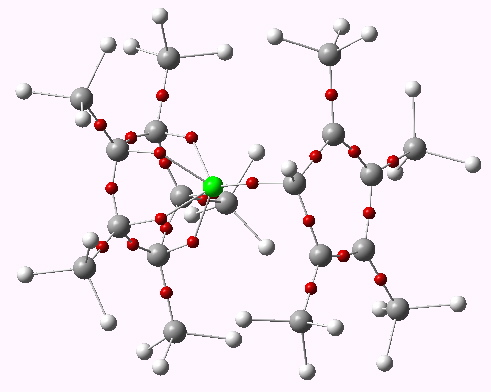
In discussing ferrocene in the previous post, I mentioned Irving Langmuir’s 1921 postulate that filled valence shells in what he called complete molecules would have magic numbers of 2, 8, 18 or 32 electrons (deriving from the sum of terms in 2[1+3+5+7]). The first two dominate organic chemistry of course, whilst the third is illustrated by […]