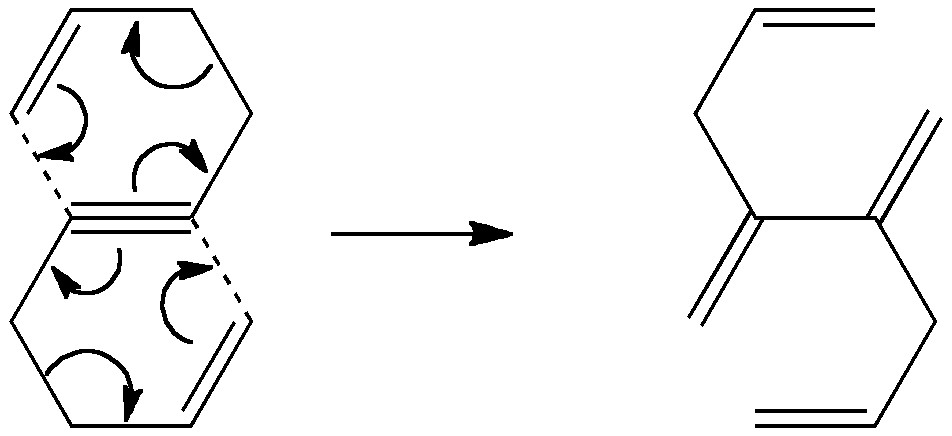
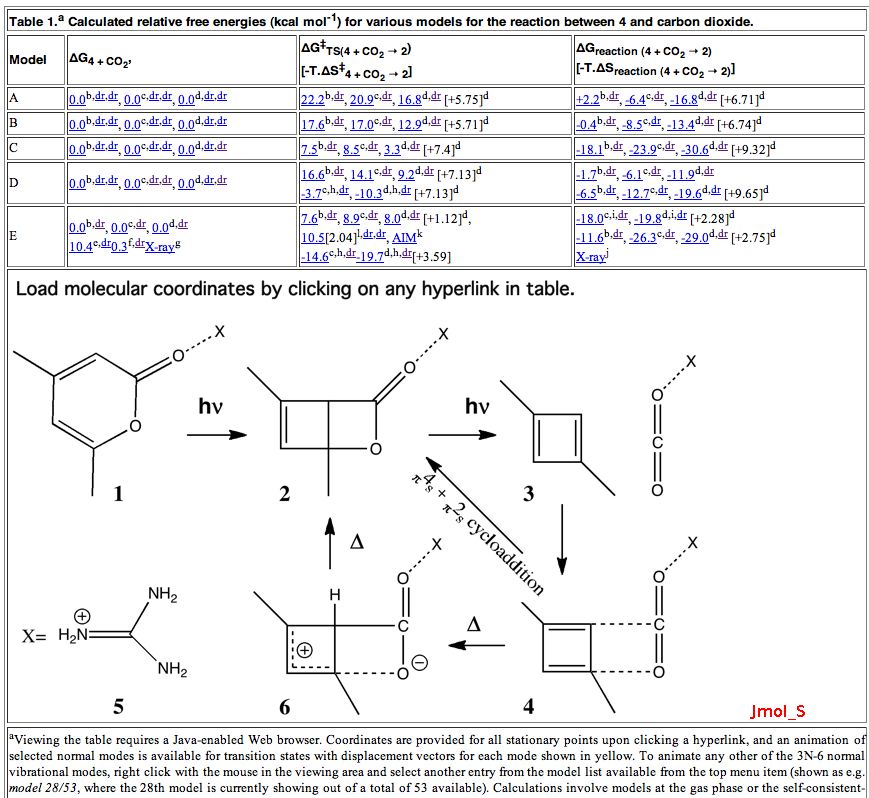
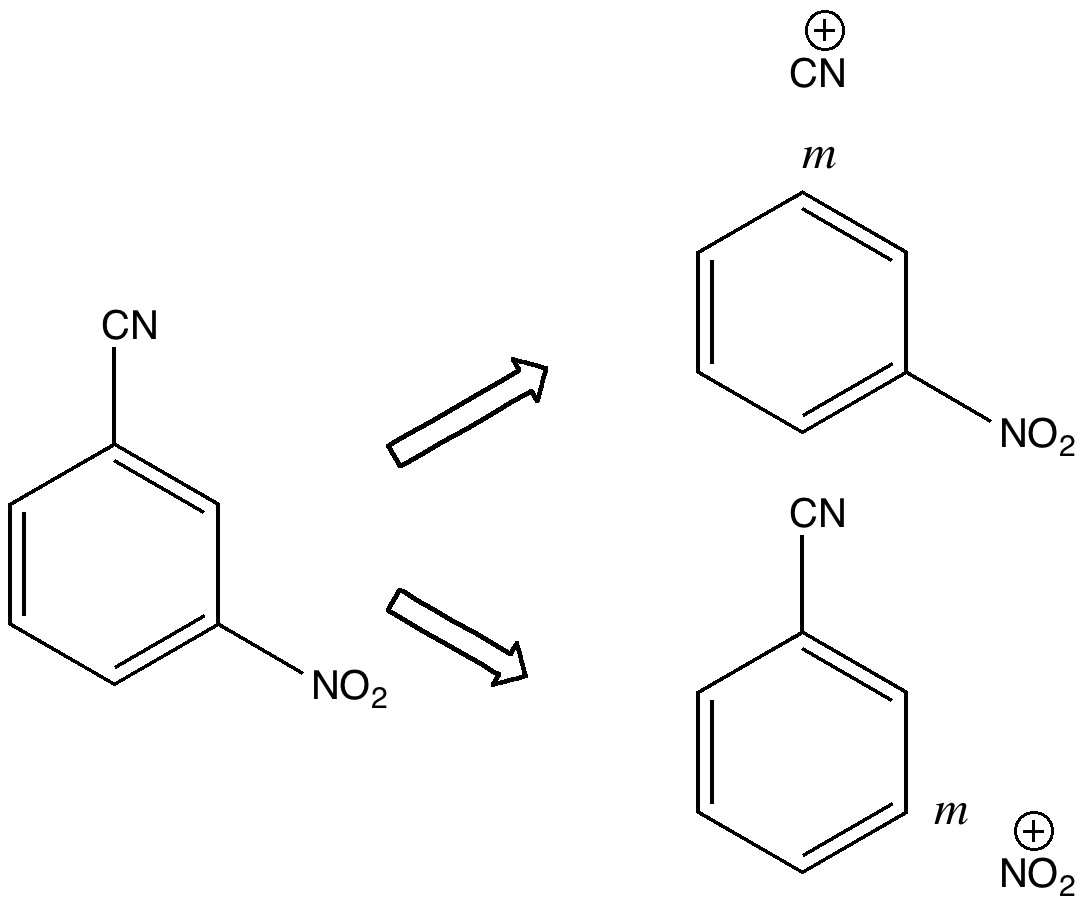
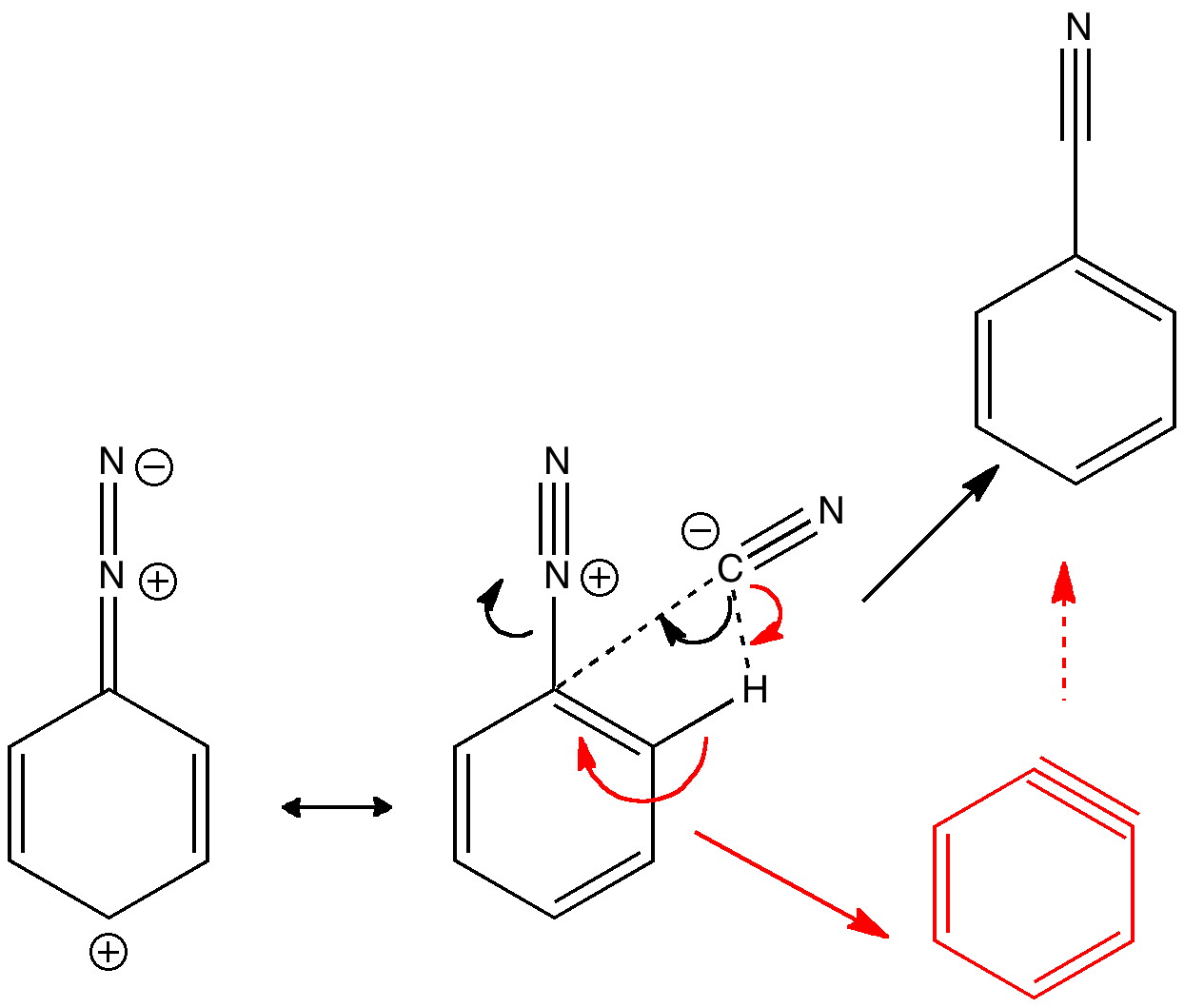
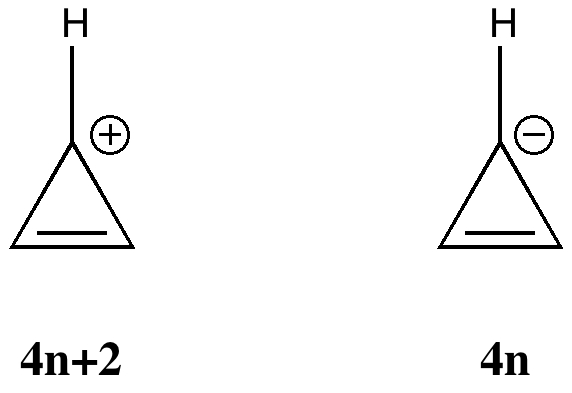
Science is about making connections. Plenty are on show in Watson and Crick’s famous 1953 article on the structure of DNA but often with the tersest of explanations. Take for example their statement “Both chains follow right-handed helices“. Where did that come from?