Werte Kolleg*innen, wir möchten Sie gerne an den noch bis 31.03.2026 laufenden Call «Let’s talk. Interviews» für die Ausgabe #48 der LIBREAS erinnern.
Rogue Scholar Posts

Resignations at Anthropic's safeguards research team and xAI, Opus 4.6 evaluation awareness, Bytedance AI video generation, US doesn't back Global AI Safety Report, "heinous crimes" and $1T selloffs

Wissenschaft ist bekanntlich eine ernste Angelegenheit. Monografien mit nüchternen Titeln, Aufsätze voller Formeln und Fußnoten prägen den Arbeitsalltag in den Fachreferaten der TIB. Und doch: Wer regelmäßig neue Titel sichtet und für den Bibliotheksbestand auswählt, weiß, dass sich zwischen all der fachlichen Strenge hin und wieder kleine Überraschungen verbergen.
The Dutch Research Council (NWO) announced recently that it has integrated ROR in its open API. Hans de Jonge, director of Open Science, tells us why and how NWO uses ROR in this latest installment of our case study series.
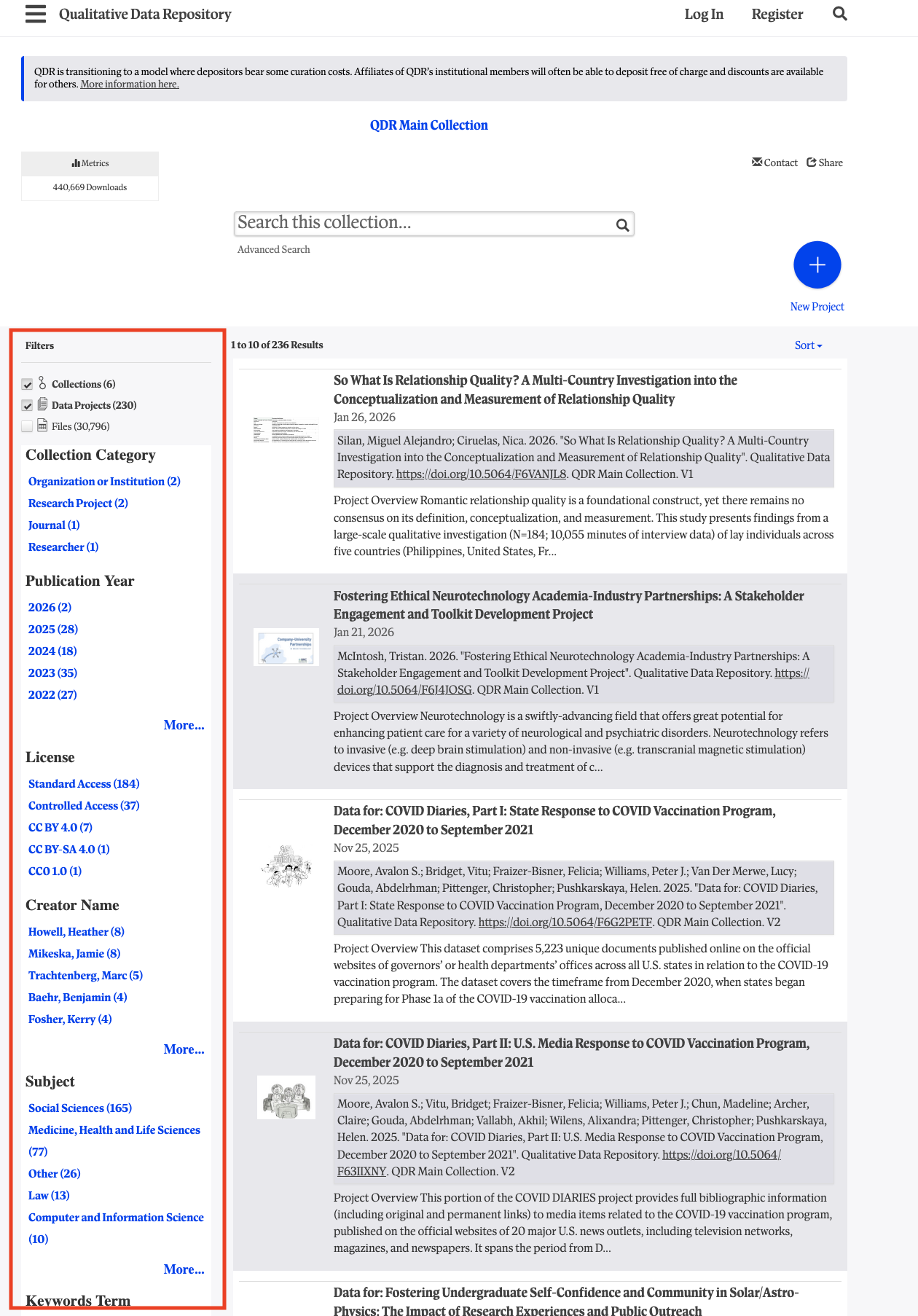
Searching and Browsing QDR with Facets DOI: https://doi.org/10.59350/w4xgx-vbc07 To mark this year’s Love Data Week, the QDR team is offering a set of three brief blog entries highlighting new features for our users and tips on how to make the most of the repository’s offerings. This is the second blog post. Finding the right materials in QDR's growing catalog is now even faster and more precise.

This is the January 2026 issue of the monthly newsletter from the Rogue Scholar science blog archive. The newsletter reports on new blogs that have joined the platform, important technical updates in Rogue Scholar infrastructure, community updates, and other news relevant to Rogue Scholar users.
Your browser does not support the audio tag. Download: PDF | EPUB | MP3 | WATCH VIDEO We are at war with death and its causes. We are building towards an infinite horizon. — Bryan Johnson A common theme in science fiction is that people of the future will live far longer than folks today. The reasoning goes something like this. Historically, human life expectancy was short — often under 30 years.
At the annual face-to-face meeting, representatives from universities, funders, national infrastructures, and research organisations reviewed key developments from the past year and agreed on a set of shared priorities for 2026.Initiated in the summer of 2024 with seven members, the network has since grown into a broad coalition, including Leiden University, Maastricht University, Utrecht University, Vrije Universiteit Amsterdam, University of
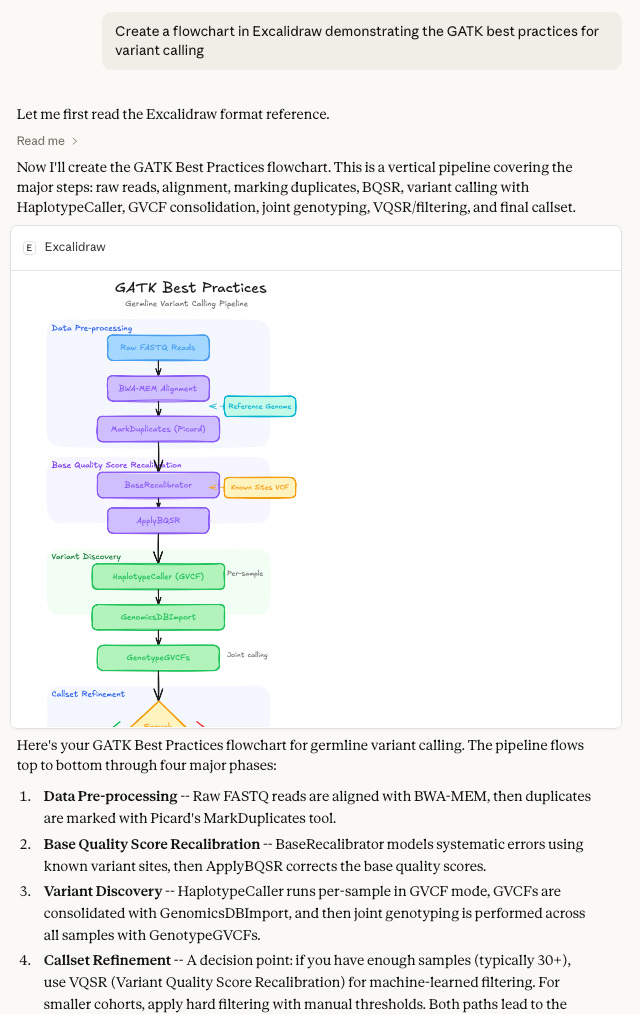
Make diagrams with draw.io using Claude project instructions or MCP, or with Excalidraw using the connector.

read this article in English Die Blogreihe „Frauen in der Wissenschaft“ stellt Frauen aus der TIB vor, die Einblicke in ihre Wege und ihre persönlichen Erfahrungen in der Wissenschaft geben. Dr. Anna-Lena Lorenz studierte Physik an der Universität Bielefeld und arbeitet heute als Community-Managerin an der TIB. Dort ist sie für den Open Research Knowledge Graph (ORGK) und den Service ORKG Ask verantwortlich.

Plant pathogens are ubiquitous in terrestrial ecosystems, but their role in these ecosystems is understudied. One aspect of their ecology that has not been studied is their influence on nutrient cycling.