In my story about one of the molecules of the year, cyclo[48]carbon, I noted that the DFT method used in the literature to model the C-C bond length alternation around the ring (OX B3LYP30) had been re-calibrated against a remeasured crystal structure of C18H18 or [18]-annulene (below) in order to reproduce the observed values for […]
Sandwich compounds are the colloquial term used for molecules where a metal atom such as an iron dication is “sandwiched” between two carbon-based rings as ligands, most commonly cyclopentadienyl anion (the “bread”) as in e.g. Ferrocene – a molecule first discovered in 1951.
The annual “Molecules of the Year” selections are available for the year 2025.
An investigation of the kinetics of the reaction between titanocene pentasulfide and sulfenyl chloride leading to the formation of the S7 allotrope of sulfur was accompanied by supporting DFT calculations which led to the conclusion that of five possible mechanisms for the reaction, the most probable corresponded to a variant of the concerted σ-bond metathesis (Scheme 1, […]
Reinvestigating the reported transition state structure of a concerted triple H-tunneling mechanism.
Substituting a deuterium isotope (2H) for a normal protium hydrogen isotope can slow the rate of a chemical reaction if this atom is involved in the reaction mode.
In the previous post, I was commenting that the transition state for borohydride reduction of a ketone contained some close contacts between the hydrogen of the borohydride and the hydrogen of water.
Part one of this topic was posted more than ten years ago. I clearly forgot about it, so belatedly, here is part 2 – dealing with the stereochemistry of the reduction of tert-butyl-cyclohexanone by borohydride in water.
In the previous post I mooted the possibility that a high energy form of the dimer of nitric oxide 1 might nonetheless be able to be detected using suitable traps (such as hydrogenation or cycloaddition). However, an interesting alternative is that this species could be trapped by nitric oxide itself.
Previously I looked at some of the properties of the mysterious dimer of nitric oxide 1 – not the known weak dimer but a higher energy form with a “triple” N≡N bond.
I started adding citations to my blog posts around 2012 using the Kcite plugin.‡ Rogue Scholar is a service that monitors registered blog sources and adds all sorts of value to the original post, including identifying such citations and creating a list of them.
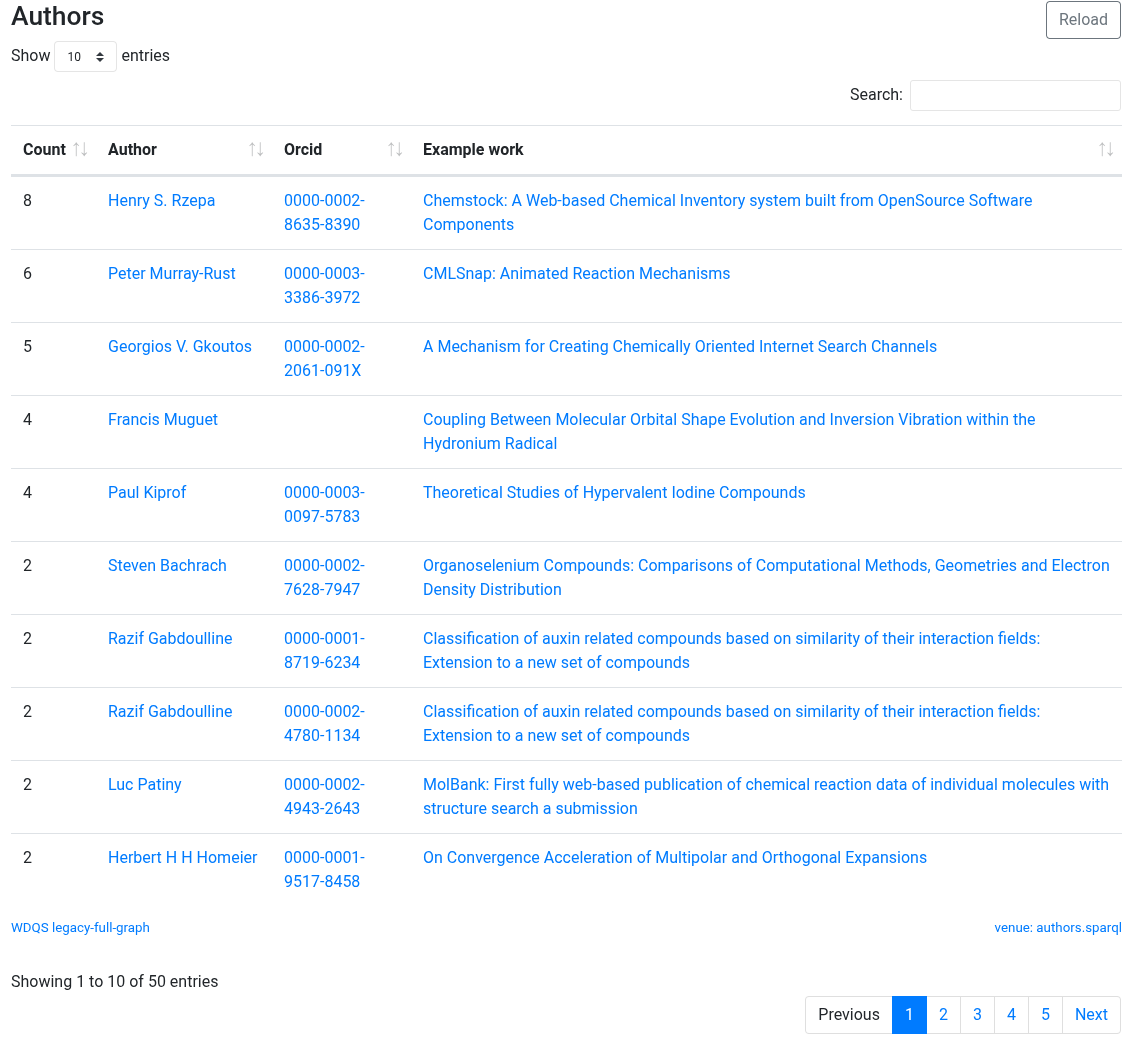
Two years ago, I posted on the topic “Internet Archeology: reviving a 2001 article published in the Internet Journal of Chemistry (IJC)”. The IJC had been founded in 1998, in part at least to “re-invent” the scholarly journal by elevating research data to being a more integrated part of the overall article, rather than as […]