The quote of the post title comes from R. B. Woodward explaining the genesis of the discovery of what are now known as the Woodward-Hoffmann rules for pericyclic reactions.
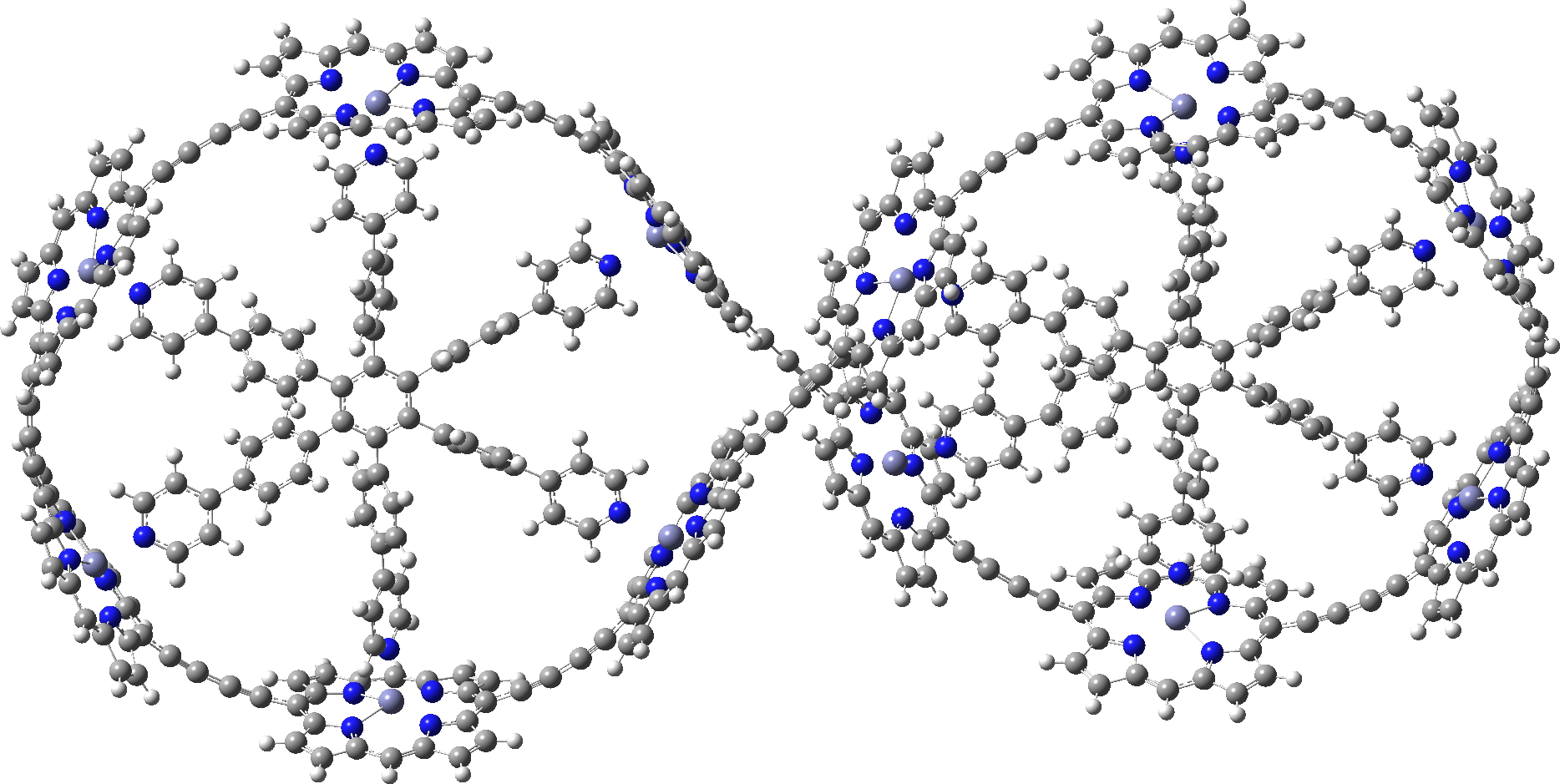
In the previous post, I showed the geometries of three large cyclic porphyrins, as part of an article on exploring the aromaticity of large 4n+2 cyclic rings. One of them had been induced into a “figure-eight” or lemniscular conformation, as shown below.
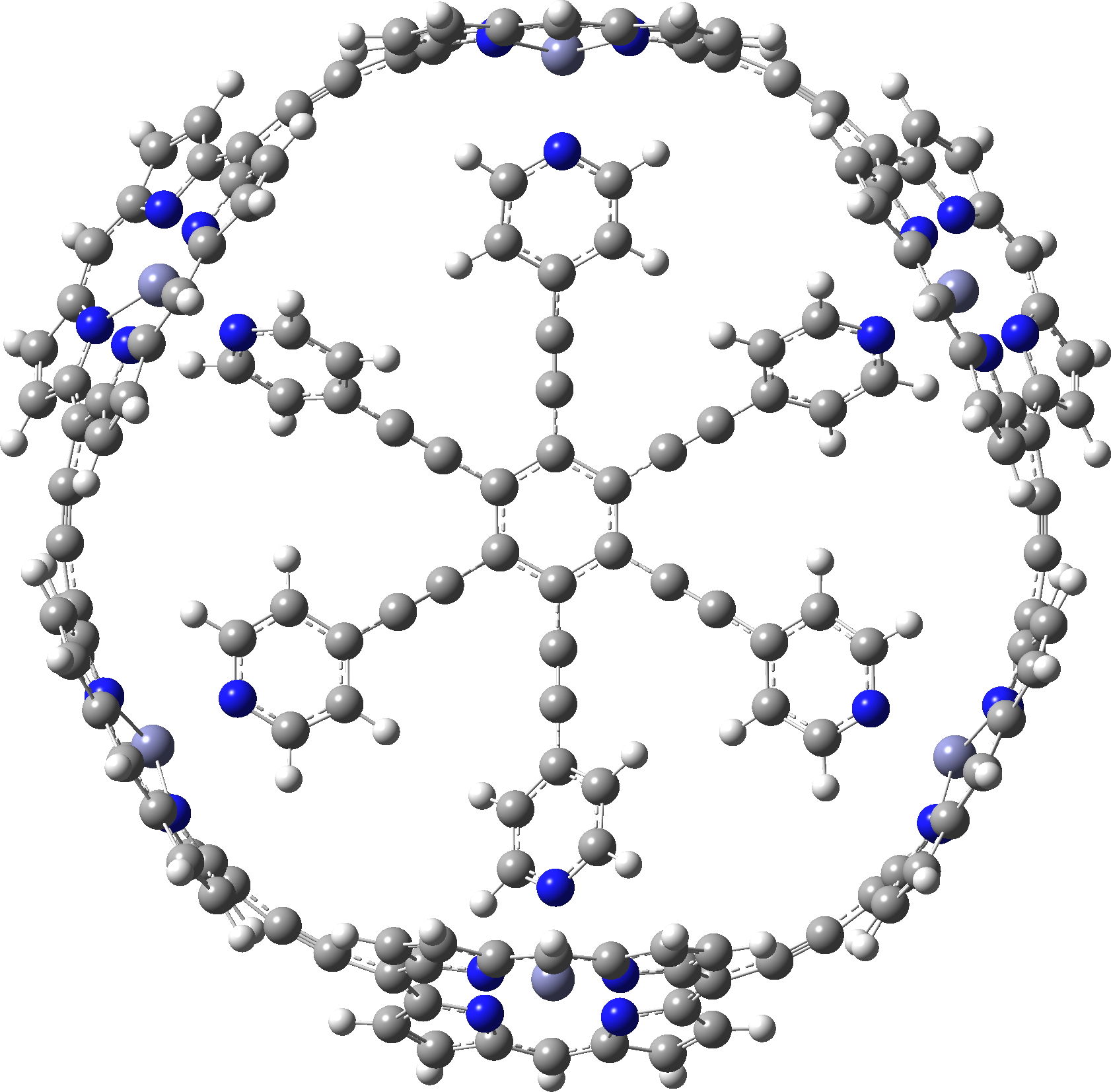
Here is another of the “large” molecules in the c&e news shortlist for molecule-of-the-year, 2020.
The title derives from an article which was shortlisted for the annual c&en molecule of the year 2020 awards (and which I occasionally cover here). In fact this year’s overall theme is certainly large molecules, the one exception being a smaller molecule with a quadruple bond to boron, a theme I have already covered here.
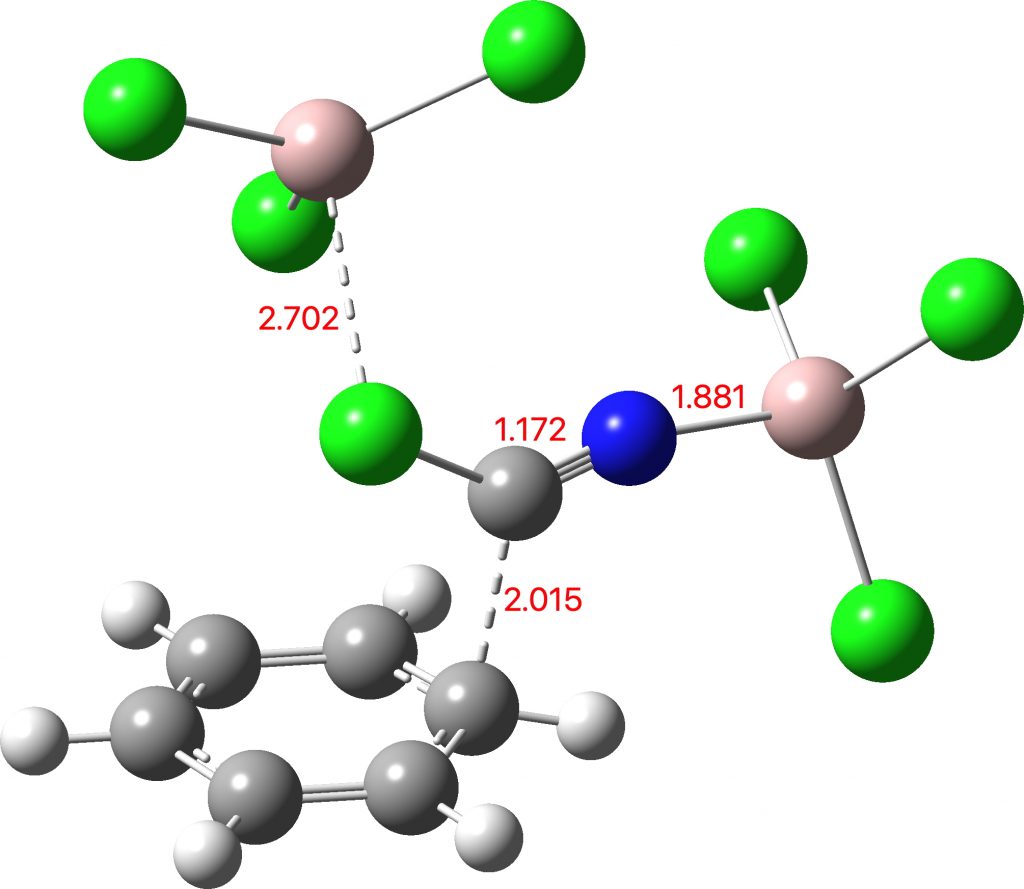
I asked the question in my previous post.
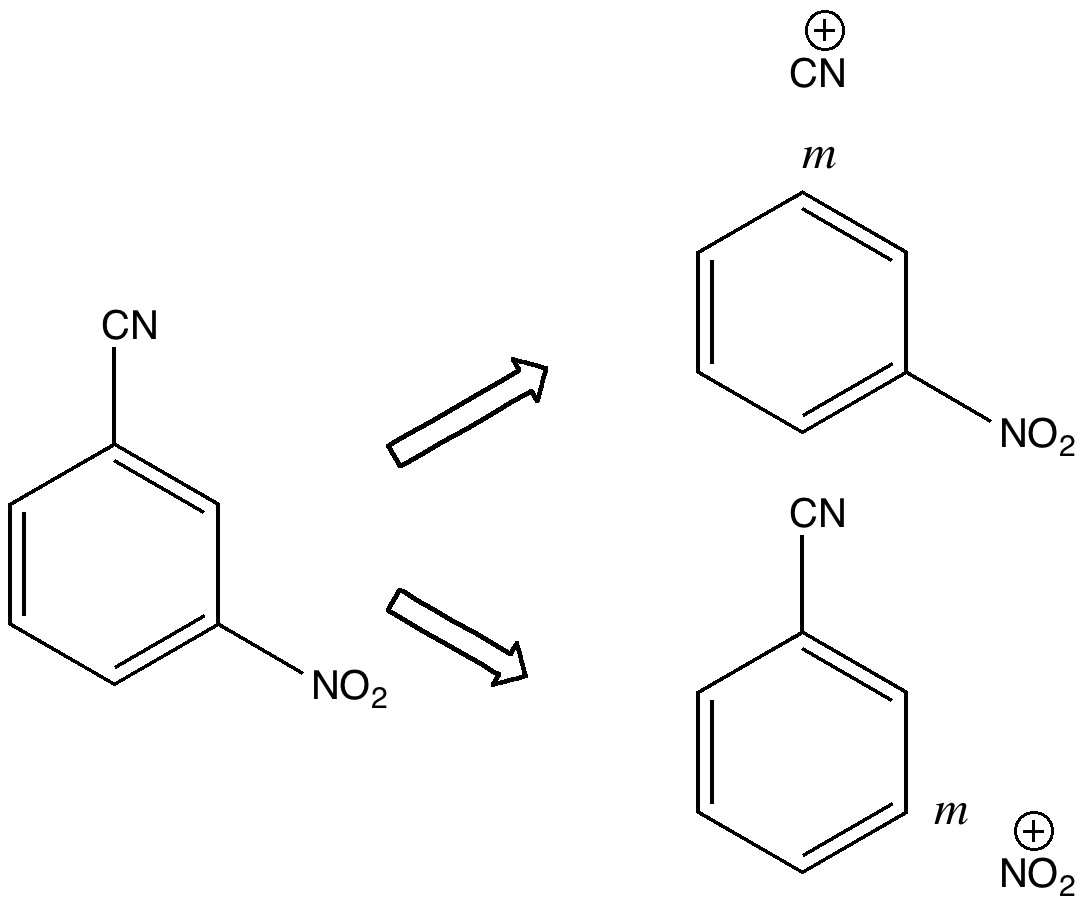
In 2010 I recounted the story of an organic chemistry tutorial, in which I asked the students the question “how would you synthesize 3-nitrobenzonitrile“.
Cyclopropenylidene must be the smallest molecule to be aromatic due to π-electrons, with just three carbon atoms and two hydrogen atoms. It has now been detected in the atmosphere of Titan, one of Saturn’s moons and joins benzene, another aromatic molecule together with the protonated version of cyclopropenylidene, C3H3+ also found there.
Way back in 2010, I was writing about an experience I had just had during an organic chemistry tutorial, which morphed into speculation as to whether a carbon atom might sustain a quadruple bond to nitrogen.

In Internet terms, 23 years ago is verging on pre-history. Much of what was happening around 1997 on the Web was still highly experimental and so its worth taking a look at some of this to see how it has survived or whether it can be “curated” into a form that would still be useful.
In an earlier post, I pondered on how the “arrow pushing” for the thermal pericyclic reactions of some annulenes (cyclic conjugated hydrocarbons) could be represented in terms of either two separate electrocyclic reactions or of one cycloaddition reaction.
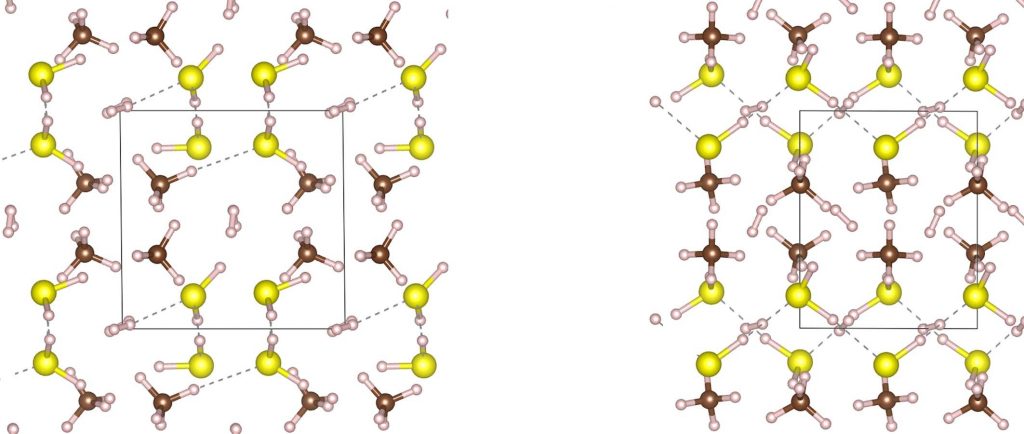
The title of this post indicates the exciting prospect that a method of producing a room temperature superconductor has finally been achived. This is only possible at enormous pressures however; >267 gigaPascals (GPa) or 2,635,023 atmospheres.