
Another occasional conference report (day 1). So why is one about “persistent identifiers” important, and particularly to the chemistry domain?

Another occasional conference report (day 1). So why is one about “persistent identifiers” important, and particularly to the chemistry domain?
I don’t normally write about the pharmaceutical industry, but I was intrigued by several posts by Derek Lowe (who does cover this area) on the topic of creating new drugs by deuterating existing ones. Thus he covered the first deuterated drug receiving FDA approval last year, having first reviewed the concept back in 2009.
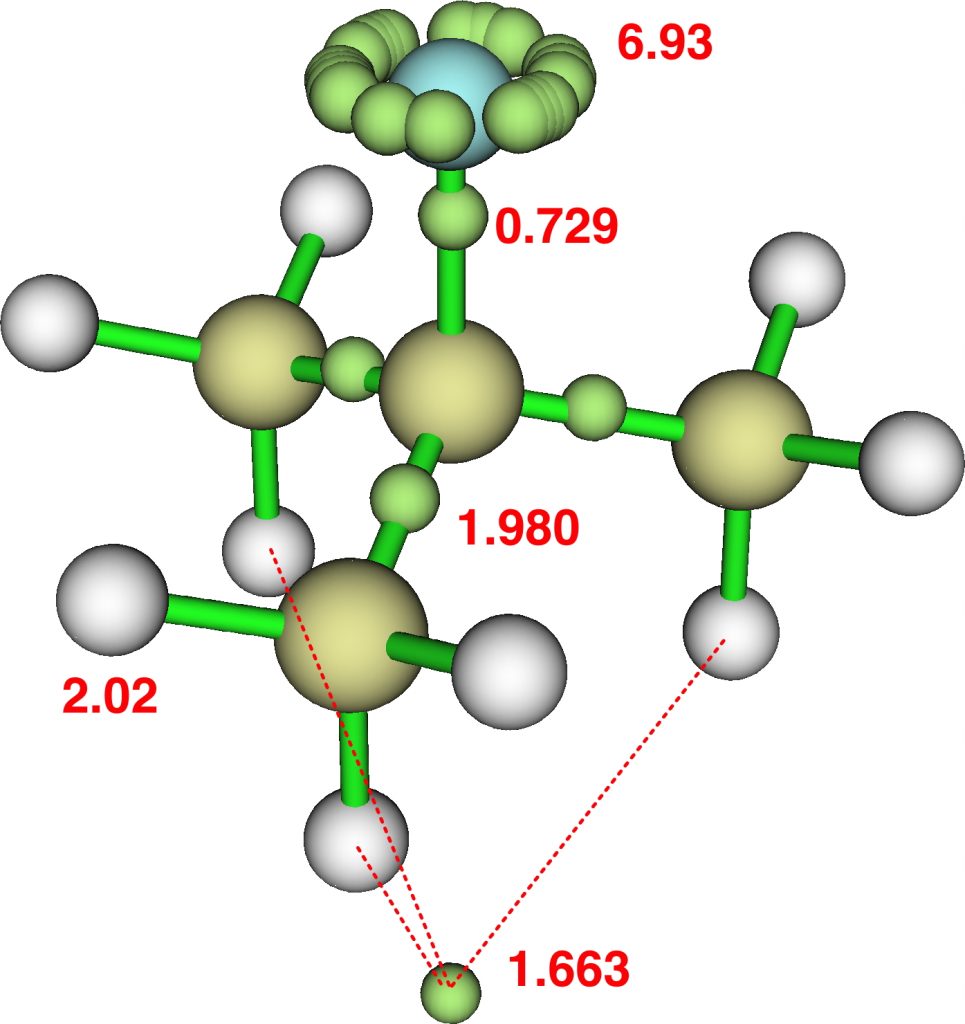
I discussed the molecule the molecule CH 3 F 2- a while back.
The title here is from an article on metalenses which caught my eye.
Recollect the suggestion that diazomethane has hypervalent character. When I looked into this, I came to the conclusion that it probably was mildly hypervalent, but on carbon and not nitrogen. Here I try some variations with substituents to see what light if any this casts.
In the previous post, I referred to a recently published review on hypervalency which introduced a very simple way (the valence electron equivalent γ) of quantifying the effect. Diazomethane was cited as one example of a small molecule exhibiting hypervalency (on nitrogen) by this measure.
A recently published review on hypervalency introduced a very simple way of quantifying the effect. One of the molecules which was suggested to be hypervalent using this method was diazomethane. Here I take a closer look.
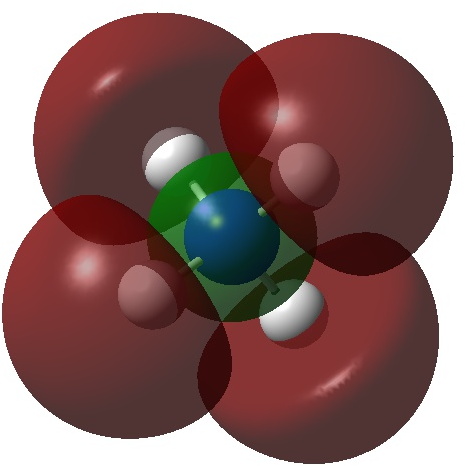
Alkalides are anionic alkali compounds containing e.g. sodide (Na – ), kalide (K – ), rubidide (Rb – ) or caeside (Cs – ). Around 90 examples can be found in the Cambridge structure database (see DOI: 10.14469/hpc/3453 for the search query and results). So what about the ammonium analogue, ammonide (NH 4 – )? A quick search of Scifinder drew a blank!
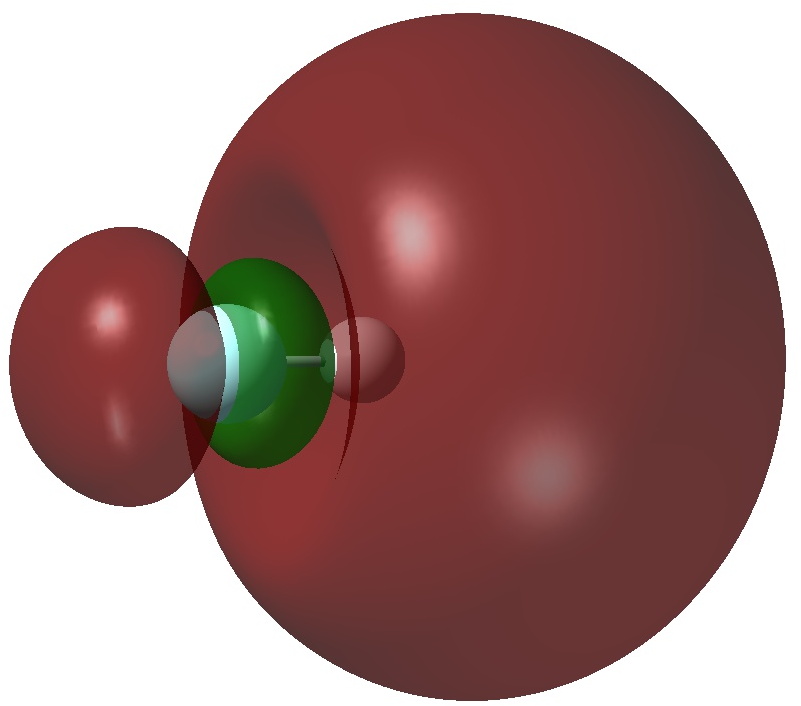
An article with the title shown above in part recently appeared. Given the apparent similarity of HF1- to CH3F1- and CH3F2-, the latter of which I introduced on this blog previously, I thought it of interest to apply my analysis to HF1-.
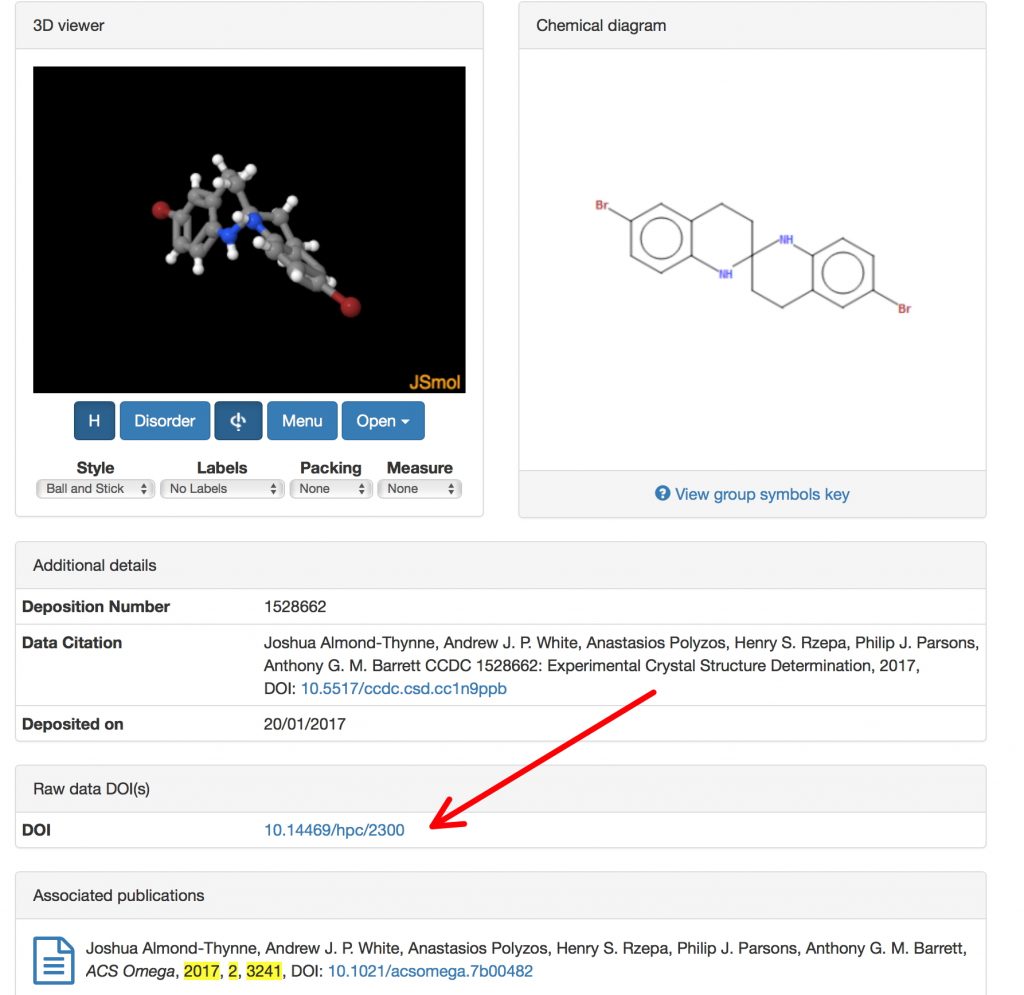
FAIR data is increasingly accepted as a description of what research data should aspire to; Findable, Accessible, Inter-operable and Re-usable, with Context added by rich metadata (and also that it should be Open). But there are two sides to data, one of which is the raw data emerging from say an instrument or software simulations […]
For around 16 years, Floyd Romesberg’s group has been exploring un-natural alternatives (UBPs) to the Watson-Crick base pairs (C-G and A-T) that form part of the genetic code in DNA.