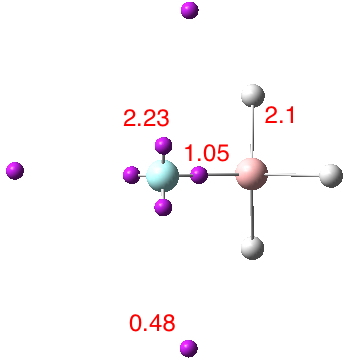
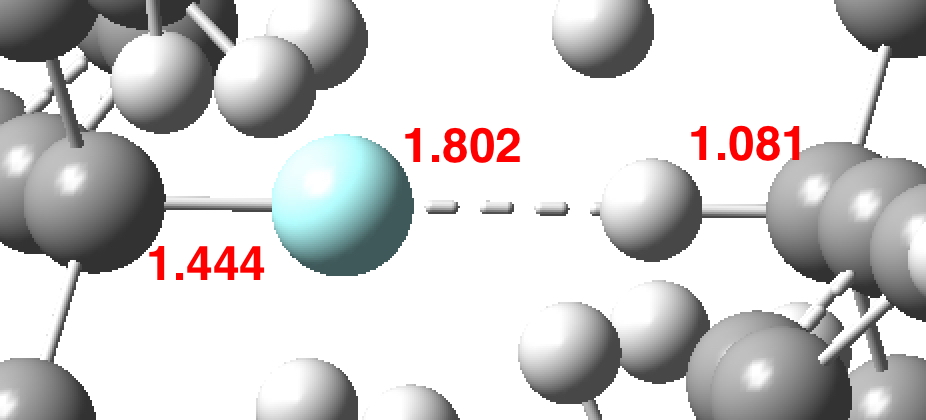
A recent article reports, amongst other topics, a computationally modelled reaction involving the capture of molecular hydrogen using a substituted borane (X=N, Y=C). The mechanism involves an initial equilibrium between React and Int1, followed by capture of the hydrogen by Int1 to form a 5-coordinate borane intermediate (Int2 below, as per Figure 11).‡ This was […]

Early in 2011, I wrote about how the diatomic molecule Be2 might be persuaded to improve upon its normal unbound state (bond order ~zero) by a double electronic excitation to a strongly bound species. I yesterday updated this post with further suggestions and one of these inspired this follow-up.
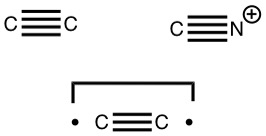
The chemical bond zoo is relatively small (the bond being a somewhat fuzzy concept, I am not sure there is an actual count of occupants). So when two new candidates come along, it is worth taking notice. I have previously noted the Chemical Bonds at the 21st Century-2017: CB2017 Aachen conference, where both were discussed.

Conferences can be intense, and this one is no exception. After five days, saturation is in danger of setting in. But before it does, I include two more (very) brief things I have learnt.
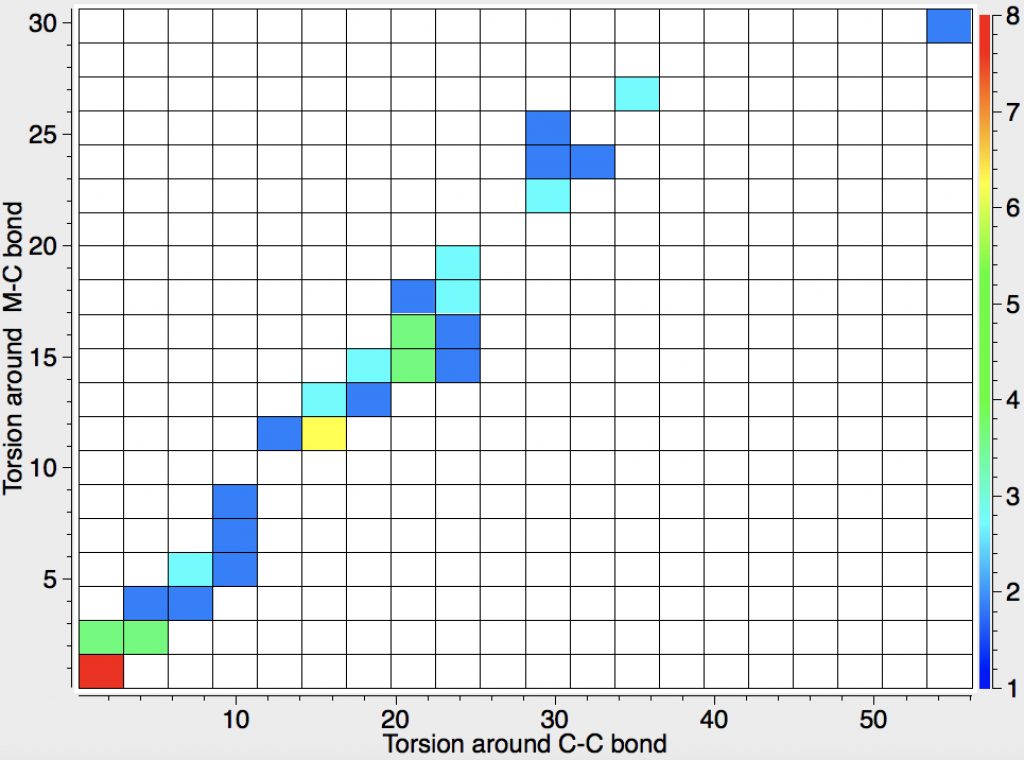
Another selection (based on my interests, I have to repeat) from WATOC 2017 in Munich. Odile Eisenstein gave a talk about predicted 13C chemical shifts in transition metal (and often transient) complexes, with the focus on metallacyclobutanes. These calculations include full spin-orbit/relativistic corrections, essential when the carbon is attached to an even slightly relativistic element.

The triennial conference is this year located in Munich. With 1500 participants and six parallel sessions, this report can give only a flavour of proceedings.
At the moment, the bond slam is something of a home from home for this blog and since much of my activity is happening there rather than here, I thought I might give you pointers to some of the topics, which are evolving, so to speak, before our very eyes.
It is always interesting to observe conference experiments taking place. The traditional model involves travelling to a remote venue, staying in a hotel, selecting sessions to attend from a palette of parallel streams and then interweaving chatting to colleagues both old and new over coffee, lunch, dinner or excursions.

Bees are having a tough time around the world. Oddly, they are surviving very well in cities. One reason are the wild flower meadows in London and for some summer relief I thought I would tell you the story of the one shown below.
There is much focus at the moment on how to ensure experimental replicability in e.g. the molecular sciences. An important aspect of that is having access to FAIR data; data which is findable, accessible, inter-operable and re-usable. One of the “gold standards” in chemistry is the data associated with crystal structures.
The effects of loading up lots of dispersion attractions (between t-butyl groups) into a compact molecule has the interesting consequence of allowing two “non-bonded” hydrogen atoms to approach to ~1.5Å of each other, thus creating the appearance of a “bond” where one normally would not be found.