A celebration of the life and work of the great chemist Paul von R. Schleyer was held this week in Erlangen, Germany. There were many fantastic talks given by some great chemists describing fascinating chemistry. Here I highlight the presentation given by Andy Streitwieser on the topic of organolithium chemistry, also a great interest of Schleyer's over the years.
Augmented reality, a superset if you like of virtual reality (VR), has really been hitting the headlines recently. Like 3D TV, its been a long time coming! Since ~1994 or earlier, there have been explorations of how molecular models can be transferred from actual reality to virtual reality using conventional computers (as opposed to highly specialised ones). It was around then that a combination of software (Rasmol) and
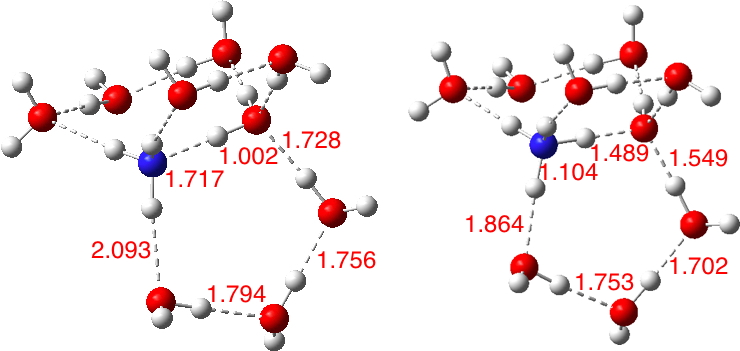
This is a corollary to the previous post ‡ exploring how many molecules are needed to ionise HCl. Here I am asking how many water molecules are required to form the ionic ammonium hydroxide from ammonia and water.
At the ACS conference, I have attended many talks these last four days, but one made some “connections” which intrigued me. I tell its story (or a part of it) here. But to start, try the following experiment. Find a Word document of .docx type on your hard drive Remove the .docx suffix and replace it with a .zip suffix. Expand as if it is an archive (it is!). A folder is created and this itself contains four further folders.
The upcoming ACS national meeting in San Diego has a CINF (chemical information division) session entitled "Global initiatives in research data management and discovery". I have highlighted here just one slide from my contribution to this session, which addresses the discovery aspect of the session. Data, if you think about it, is rarely discoverable other than by intimate association with a narrative or journal article.
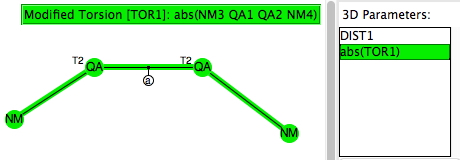
The upcoming ACS national meeting in San Diego has a CHED (chemical education division) session entitled Implementing Discovery-Based Research Experiences in Undergraduate Chemistry Courses. I had previously explored what I called extreme gauche effects in the molecule F-S-S-F. Here I take this a bit further to see what else can be discovered about molecules containing bonds between group 16 elements (QA= O, S, Se, Te).
At the precise moment I write this, there is information about 108,230,950 organic and inorganic chemical substances from the World's disclosed chemistry.

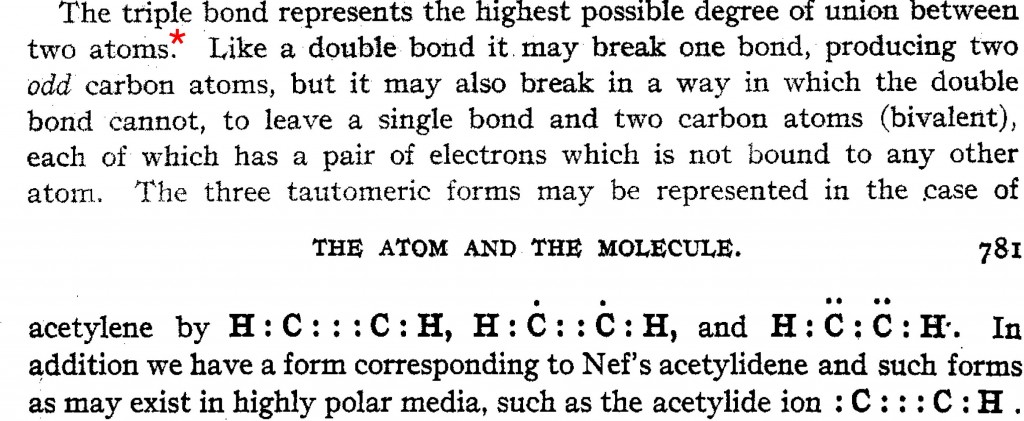
Hypervalency is defined as a molecule that contains one or more main group elements formally bearing more than eight electrons in their valence shell. One example of a molecule so characterised was CLi 6 [cite]10.1038/355432a0[/cite] where the description "“ carbon can expand its octet of electrons to form this relatively stable molecule ” was used.
The phenomenon of bond stretch isomerism , two isomers of a compound differing predominantly in just one bond length, is one of those chemical concepts that wax and occasionally wane.[cite]10.1016/S1631-0748(02)01380-2[/cite] Here I explore such isomerism for the elements Ge, Sn and Pb. In one earlier post, I noted a form of bond stretch isomerism that can arise from a Jahn-Teller distortion ending in two different geometries
The geometry of cyclo-octatetraenes differs fundamentally from the lower homologue benzene in exhibiting slow (nuclear) valence bond isomerism rather than rapid (electronic) bond-equalising resonance.
I attended the first (of a proposed five) workshops organised by LEARN (an EU-funded project that aims to .. .Raise awareness in research data management (RDM) issues & research policy ) on Friday. Here I give some quick bullet points relating to things that caught my attention and or interest. The program (and Twitter feed) can be found at https://learnrdm.wordpress.com where other's comments can also be seen.