I attended the first (of a proposed five) workshops organised by LEARN (an EU-funded project that aims to .. .Raise awareness in research data management (RDM) issues & research policy ) on Friday. Here I give some quick bullet points relating to things that caught my attention and or interest. The program (and Twitter feed) can be found at https://learnrdm.wordpress.com where other's comments can also be seen.
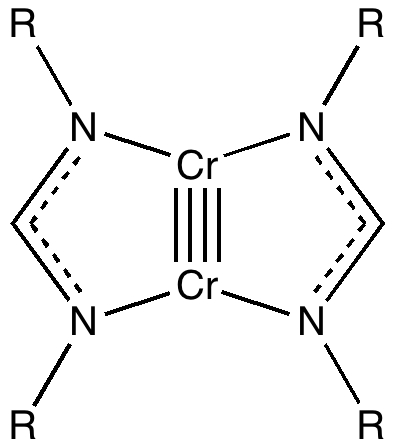
Six years ago, I posted on the nature of a then recently reported[cite]10.1002/anie.200803859[/cite] Cr-Cr quintuple bond. The topic resurfaced as part of the discussion on a more recent post on NSF 3 , and a sub-topic on the nature of the higher order bonding in C 2 . The comment made a connection between that discussion and the Cr-Cr bond alluded to above.
The original strategic objective of my PhD researches in 1972-74 was to explore how primary kinetic hydrogen isotope effects might be influenced by the underlying structures of the transition states involved. Earlier posts dealt with how one can construct quantum-chemical models of these transition states that fit the known properties of the reactions.
The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF 3 has proved perennially popular. So here is a follow-up on another little molecue, F 3 SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis. This is as follows: Six valence electrons on the central S atom.
Earlier I explored models for the heteroaromatic electrophilic protiodecarboxylation of an 3-substituted indole, focusing on the role of water as the proton transfer and delivery agent. Next, came models for both water and the general base catalysed ionization of indolinones.
This is the third and final study deriving from my Ph.D.[cite]10.1039/P29750001822[/cite]. The first two topics dealt with the mechanism of heteroaromatic electrophilic attack using either a diazonium cation or a proton as electrophile, followed by either proton abstraction or carbon dioxide loss from the resulting Wheland intermediate.
In May 2015, the EPSRC funding council in the UK required researchers to publish the outcomes of the funded work to include an OA (open access) version of the narrative and to cite the managed research data used to support the research with a DOI (digital object identifier). I was discussing these aspects with a senior manager (research outcomes) at the EPSRC and he asked me to provide some examples from my area of chemistry;
Another mechanistic study we started in 1972[cite]10.1039/P29770000281[/cite] is here 40+ years on subjected to quantum mechanical scrutiny.
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then.
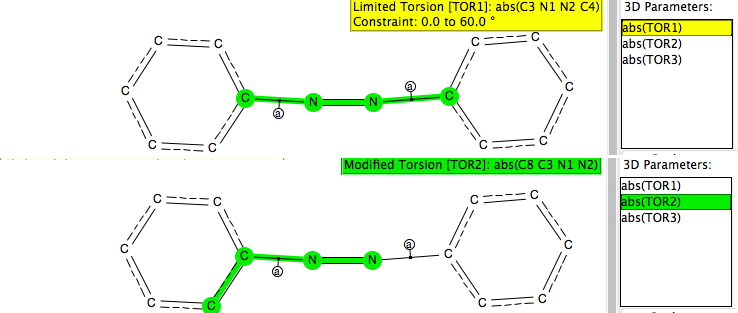
This question was posted on the CCL (computational chemistry list) by John McKelvey. Here, I give an answer in the form of a search of the CSD (crystal structure database). I was not sure if the question related purely to the geometries obtained using computational methods or to comparisons with experimentally determined structures. Or indeed whether it related to azobenzene specifically or to azobenzenes in general.
You might have noticed the occasional reference here to the upcoming centenary of the publication of Gilbert N. Lewis’ famous article entitled “ The atom and the molecule ”.[cite]10.1021/ja02261a002[/cite] A symposium exploring his scientific impact and legacy will be held in London on March 23, 2016, exactly 70 years to the day since his death. A list of the speakers and their titles is shown below;