A fascinating re-examination has appeared of a reaction first published in 1960 by Wittig and then repudiated by him in 1964 since it could not be replicated by a later student.
How does an anaesthetic work?
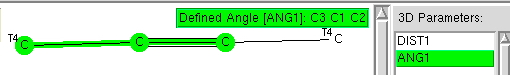
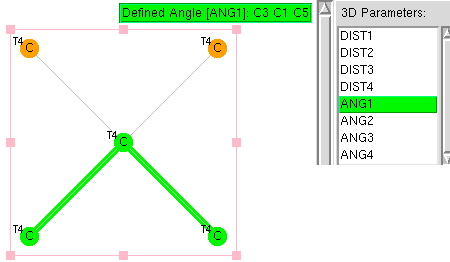
Previously, I explored deviation from ideal tetrahedral arrangements of four carbon ligands around a central (sp3) carbon using crystal structures. Now it is the turn of digonal (sp1) and trigonal (sp2) carbons.
An article entitled "Four Decades of the Chemistry of Planar Hypercoordinate Compounds" was recently reviewed by Steve Bacharach on his blog, where you can also see comments.
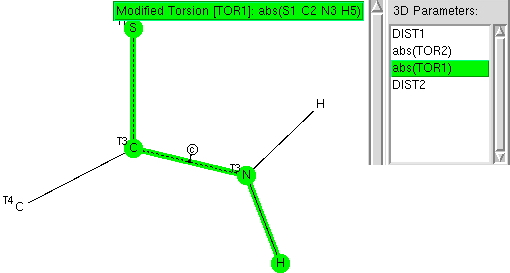
The previous post explored the structural features of amides. Here I compare the analysis with that for the closely related thioamides.
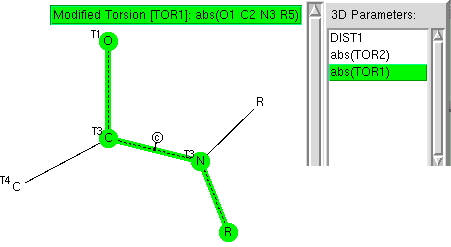
The π-resonance in amides famously helped Pauling to his proposal of a helical structure for proteins. Here I explore some geometric properties of amides related to the C-N bond and the torsions about it.
The first conference devoted to scientific uses of Wikipedia has just finished; there was lots of fascinating stuff but here I concentrate on one report that I thought was especially interesting. To introduce it, I need first to introduce WikiData. This is part of the WikiMedia ecosystem, and one of the newest.

Most visitors to London use the famous underground trains (the “tube”) or a double-decker bus to see the city (one can also use rivers and canals). So I thought, during the tourism month of August, I would show you an alternative overground circumnavigation of the city using the metaphor of benzene.
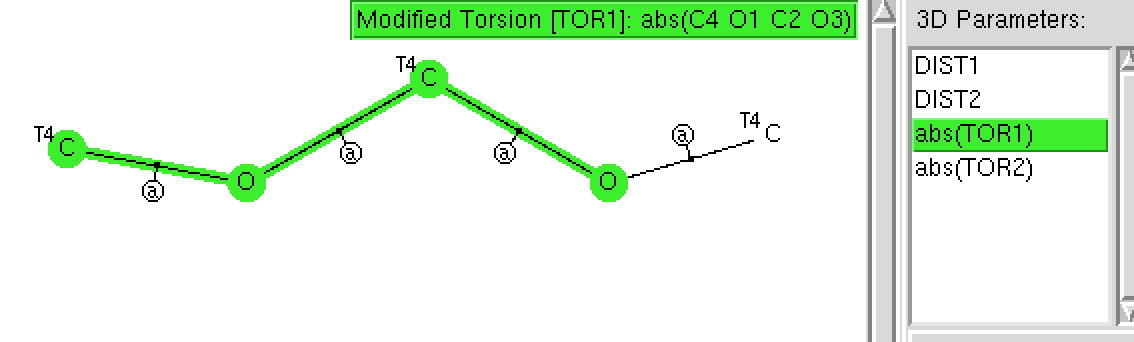
The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs.
Previously, I showed how conjugation in dienes and diaryls can be visualised by inspecting bond lengths as a function of torsions. Here is another illustration, this time of the mesomeric resonance on a benzene ring induced by an electron donating substituent (an amino group) or an electron withdrawing substituent (cyano).
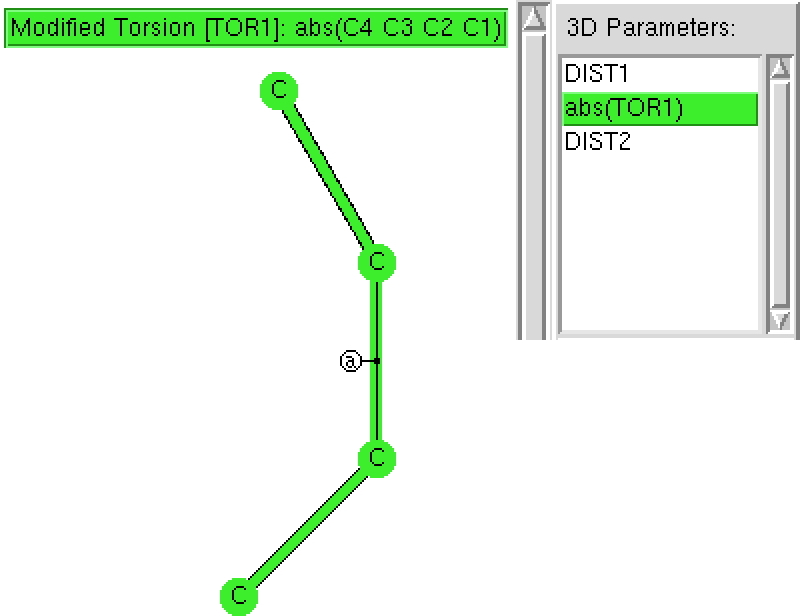
Here is another exploration of simple chemical concepts using crystal structures. Consider a simple diene: how does the central C-C bond length respond to the torsion angle between the two C=C bonds?