
Many moons ago, when I was a young(ish) lecturer, and much closer in time to my laboratory roots of organic synthesis, I made some chemistry videos. One of these has resurfaced, somewhat (to me at least) unexpectedly.

Many moons ago, when I was a young(ish) lecturer, and much closer in time to my laboratory roots of organic synthesis, I made some chemistry videos. One of these has resurfaced, somewhat (to me at least) unexpectedly.
In a time of change, we often do not notice that Δ = ∫δ. Here I am thinking of network bandwidth, and my personal experience of it over a 46 year period.
A few years ago, we published an article which drew a formal analogy between chemistry and iTunes (sic). iTunes was the first really large commercial digital music library, and a feature under-the-skin was the use of meta-data to aid discoverability of any of the 10 million (26M in 2013) or so individual items in the store.‡ […]
This is a follow-up to comment posted by Ryan, who asked about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC): “In Van Leusen, it (the isocyanide) acts as an electrophile”. The Wikipedia article (recently updated by myself) shows nucleophilic attack by an oxy-anion on the carbon of the C≡N group, […]
The title of this post comes from a comment posted by Ryan, who asks about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC) in two named reactions, Van Leusen and Ugi FCR.
Here is another example gleaned from that Woodward essay of 1967 (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249), where all might not be what it seems.
Sometimes the originators of seminal theories in chemistry write a personal and anecdotal account of their work. Niels Bohr was one such and four decades later Robert Woodward wrote “The conservation of orbital symmetry” (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249;
In the preceding post, I introduced Dewar’s π-complex theory for alkene-metal compounds, outlining the molecular orbital analysis he presented, in which the filled π-MO of the alkene donates into a Ag+ empty metal orbital and back-donation occurs from a filled metal orbital into the alkene π* MO. Here I play a little “what if” game with this […]
The period 1951–1954 was a golden one for structural chemistry; proteins, DNA, Ferrocene (1952) and the one I discuss here, a bonding model for Zeise’s salt (3).
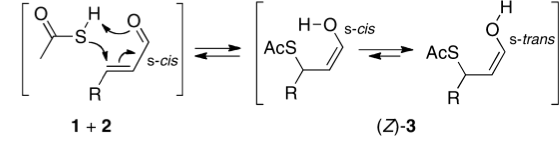
Lukas, who occasionally comments on this blog, sent me the following challenge. In a recent article he had proposed that the stereochemical outcome (Z) of reaction between a butenal and thioacetic acid as shown below arose by an unusual concerted cycloaddtion involving an S-H bond.
The previous post described how the acid catalysed ring opening of propene epoxide by an alcohol (methanol) is preceded by pre-protonation of the epoxide oxygen to form a “hidden intermediate” on the concerted intrinsic reaction pathway to ring opening.