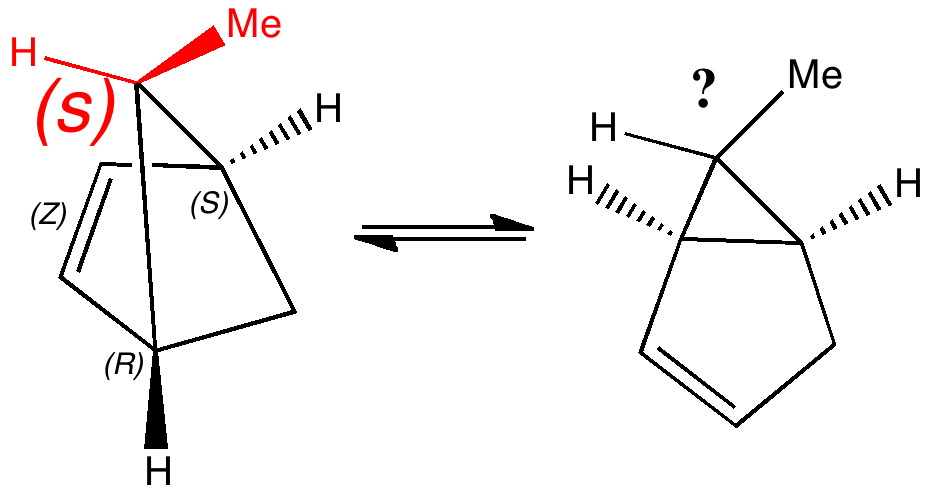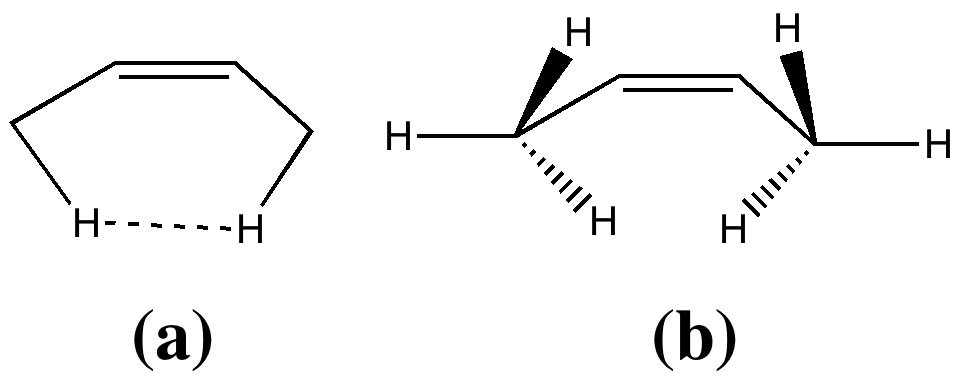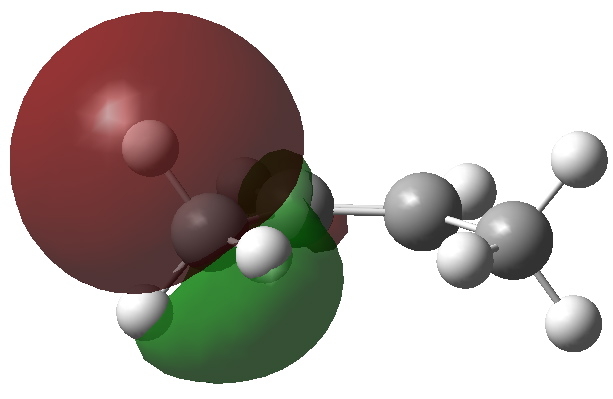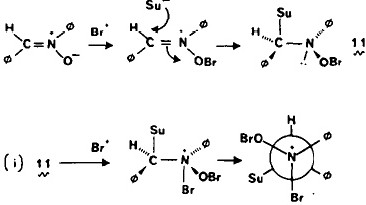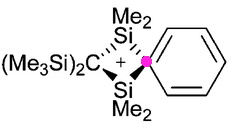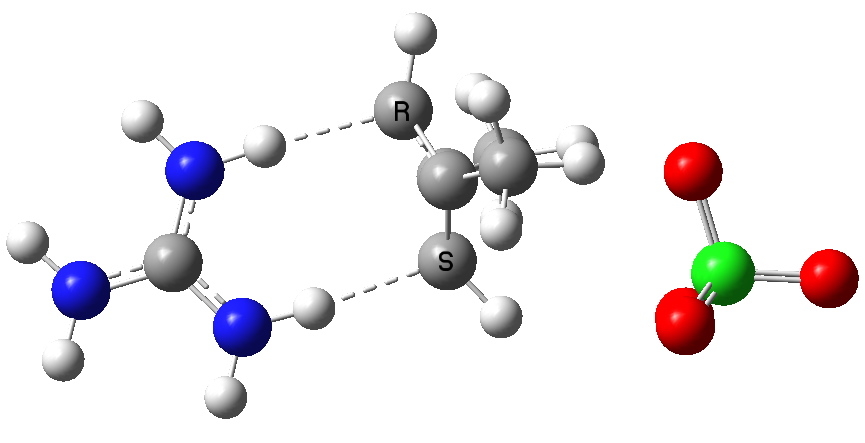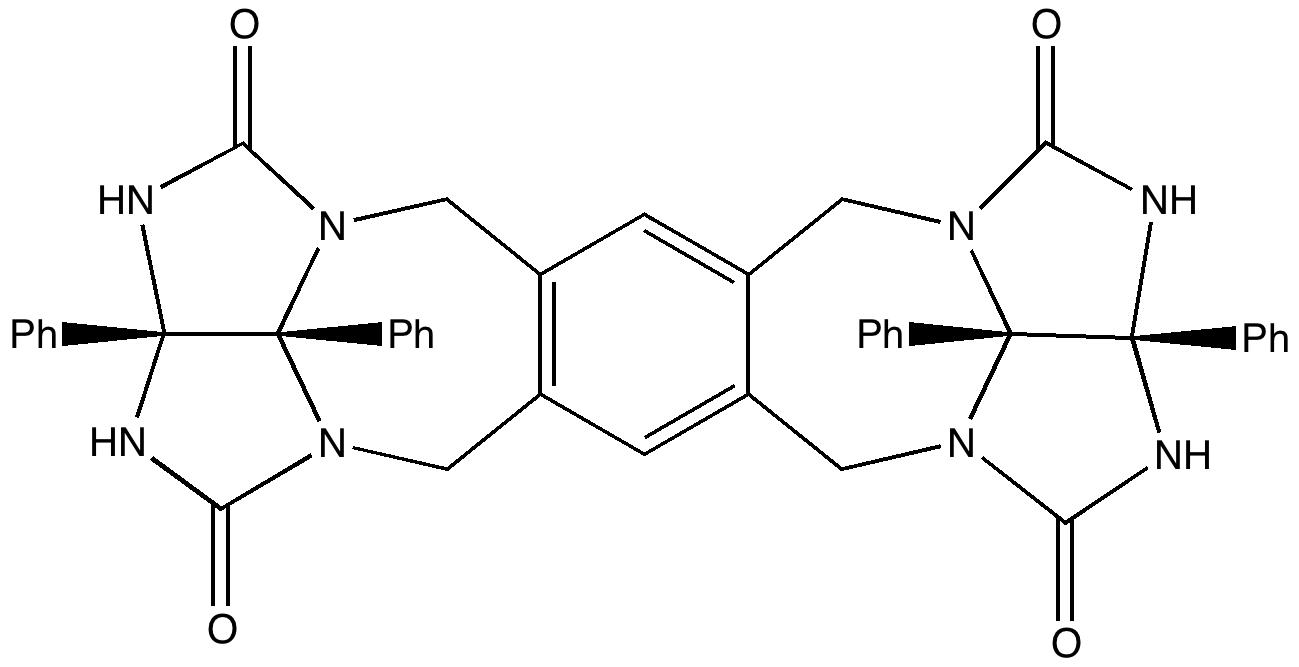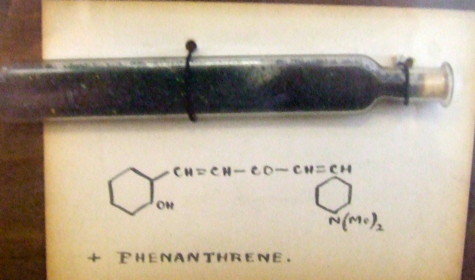
Organic chemists have been making (more or less pure) molecules for the best part of 180 years. Occasionally, these ancient samples are unearthed in cupboards, and then the hunt for their origin starts. I have previously described tracking down the structure of a 120 year-old sample of a naphthalene derivative.