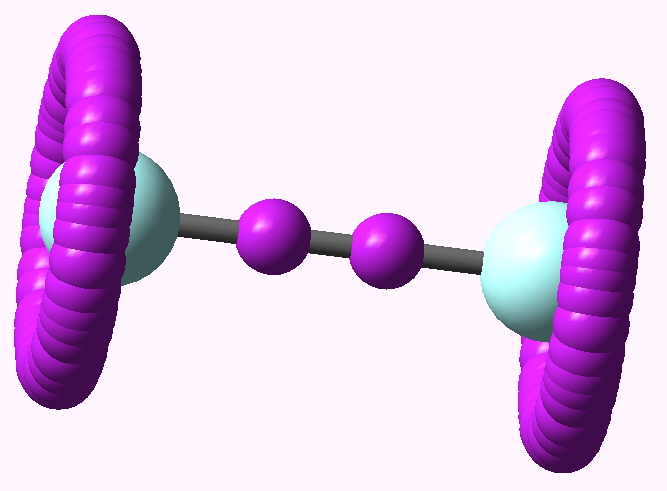
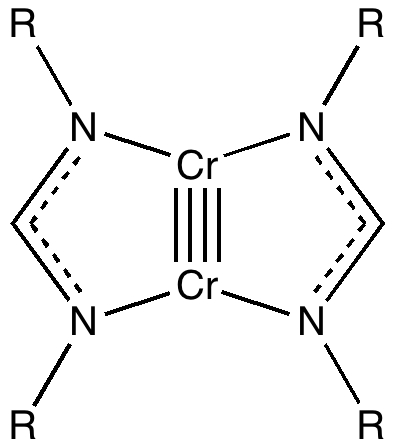
Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found […]