Scientists write blogs for a variety of reasons.
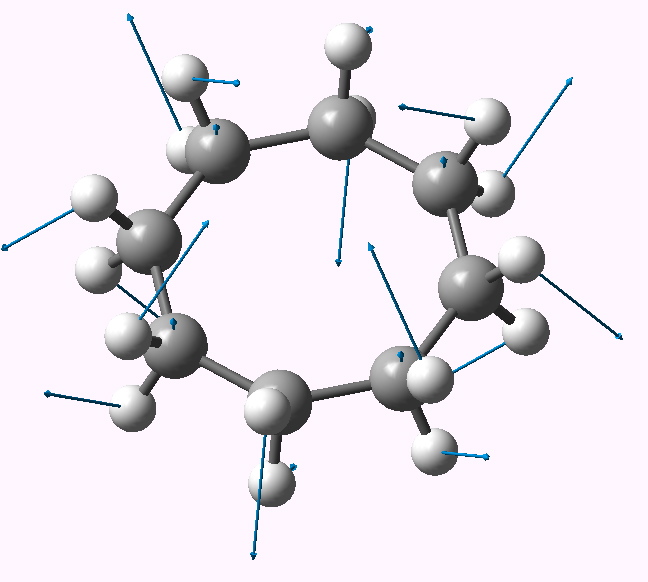
In the previous post, I suggested that inspecting the imaginary modes of planar cyclohexane might be a fruitful and systematic way in which at least parts of the conformational surface of this ring might be probed. Here, the same process is conducted for cyclo-octane.
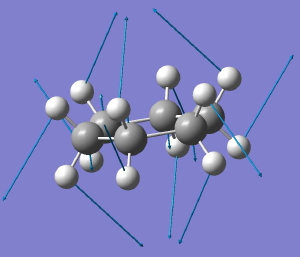
Like benzene, its fully saturated version cyclohexane represents an icon of organic chemistry. By 1890, the structure of planar benzene was pretty much understood, but organic chemistry was still struggling somewhat to fully embrace three rather than two dimensions.

So ingrained is the habit to think of a bond as a simple straight line connecting two atoms, that we rarely ask ourselves if they are bent, and if so, by how much (and indeed, does it matter?). Well Hursthouse, Malik, and Sales, as long ago as 1978, asked just such a question about the […]

A Semantic blog is one in which the system at least in part understands about (some of the) concepts and topics that are in the content.
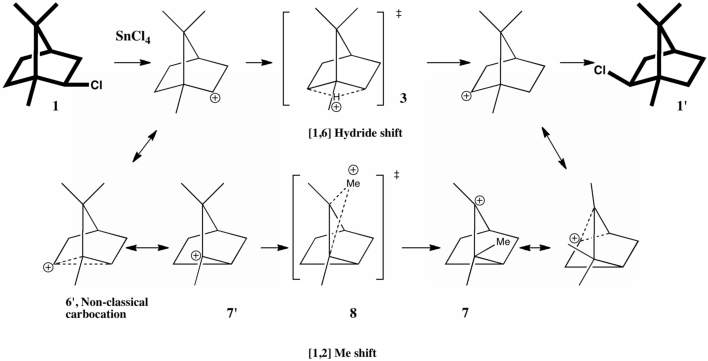
The scheme below illustrates one of the iconic reactions in organic chemistry. It is a modern representation of Meerwein’s famous experiment from which he inferred a carbocation intermediate, deduced from studying the rate of enantiomerization of isobornyl chloride when treated with the Lewis acid SnCl4.
After around 40 posts here, I decided to take a look at the whole effort and ask some questions.

In the previous post, the molecule F 3 S-C≡SF 3 was found to exhibit a valence bond isomerism, one of the S-C bonds being single, the other triple, and with a large barrier (~31 kcal/mol, ν 284 i cm -1 ) to interconversion of the two valence-bond forms. So an interesting extension of this phenomenon is shown below: A cyclic form of the SCS Motif.

A previous post posed the question; during the transformation of one molecule to another, what is the maximum number of electron pairs that can simultaneously move either to or from any one atom-pair bond as part of the reaction?

Clar islands are found not so much in an ocean, but in a type of molecule known as polycyclic aromatic hydrocarbons (PAH). One member of this class, graphene, is attracting a lot of attention recently as a potential material for use in computer chips.

In the previous two posts, a strategy for tuning the nature of the CS bond in the molecule HO-S≡C-H was developed, based largely on the lone pair of electrons identified on the carbon atom.