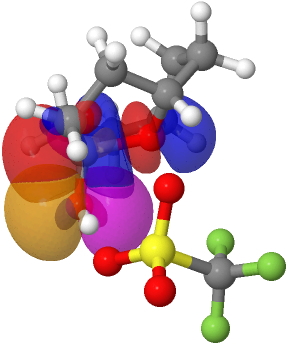
The ionization of a C-X bond (X=halogen) to form what we call a carbocation and which is known as the SN-1 reaction goes way back in the history of chemistry. Julius Steglitz was probably the first person to suggest such an ionization, back in 1899 (Steglitz, J.; Am. Chem.