Earlier this year, Molnupiravir hit the headlines as a promising antiviral drug.
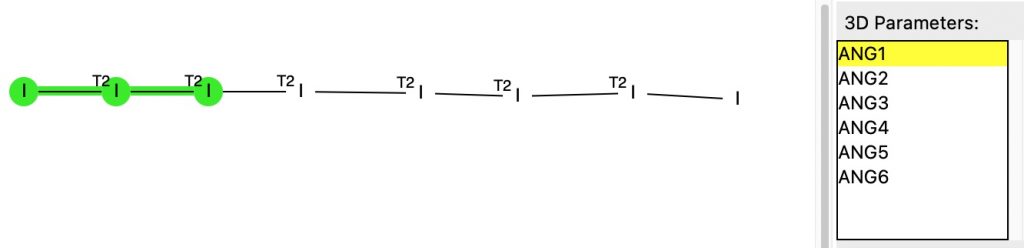
The previous post described the fascinating 170-year history of a crystalline compound known as Herapathite and its connection to the mechanism of the Finkelstein reaction via the complex of Na+I2– (or Na22+I42-). Both compounds exhibit (approximately) linear chains of iodine atoms in their crystal structures, a connection which was discovered serendipitously.
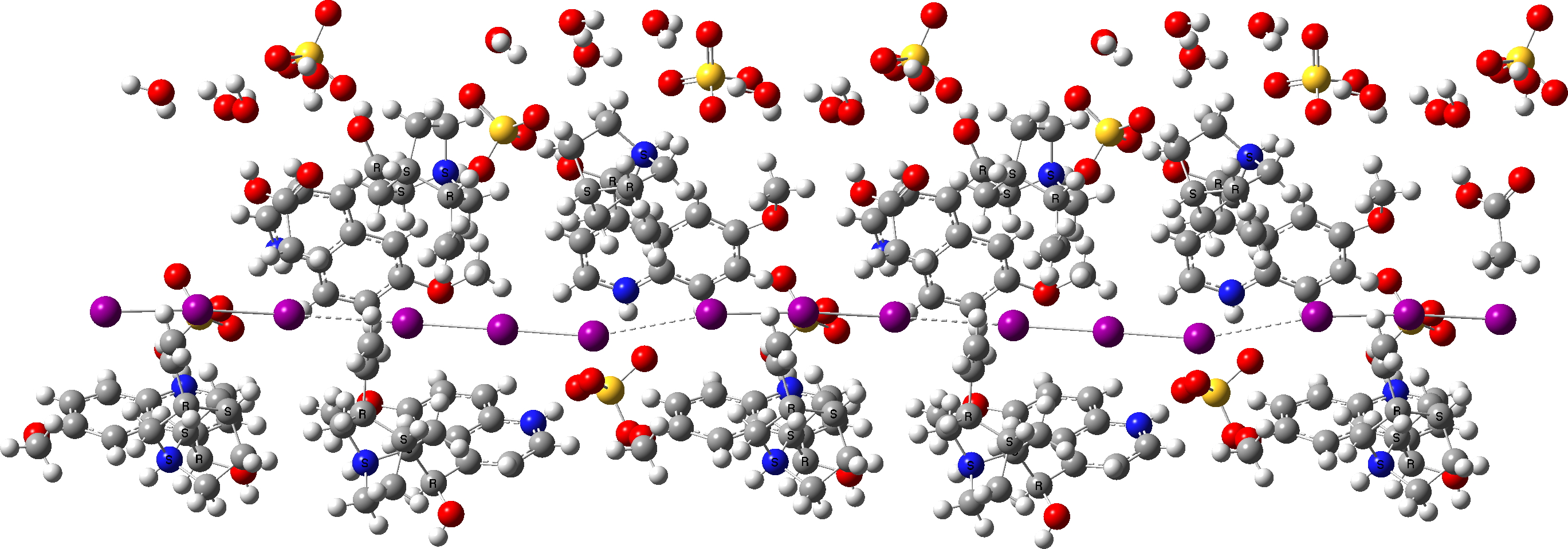
On October 13, 2021, the historical group of the Royal Society of Chemistry organised a symposium celebrating ~150 years of the history of (molecular) chirality. We met for the first time in person for more than 18 months and were treated to a splendid and diverse program about the subject.
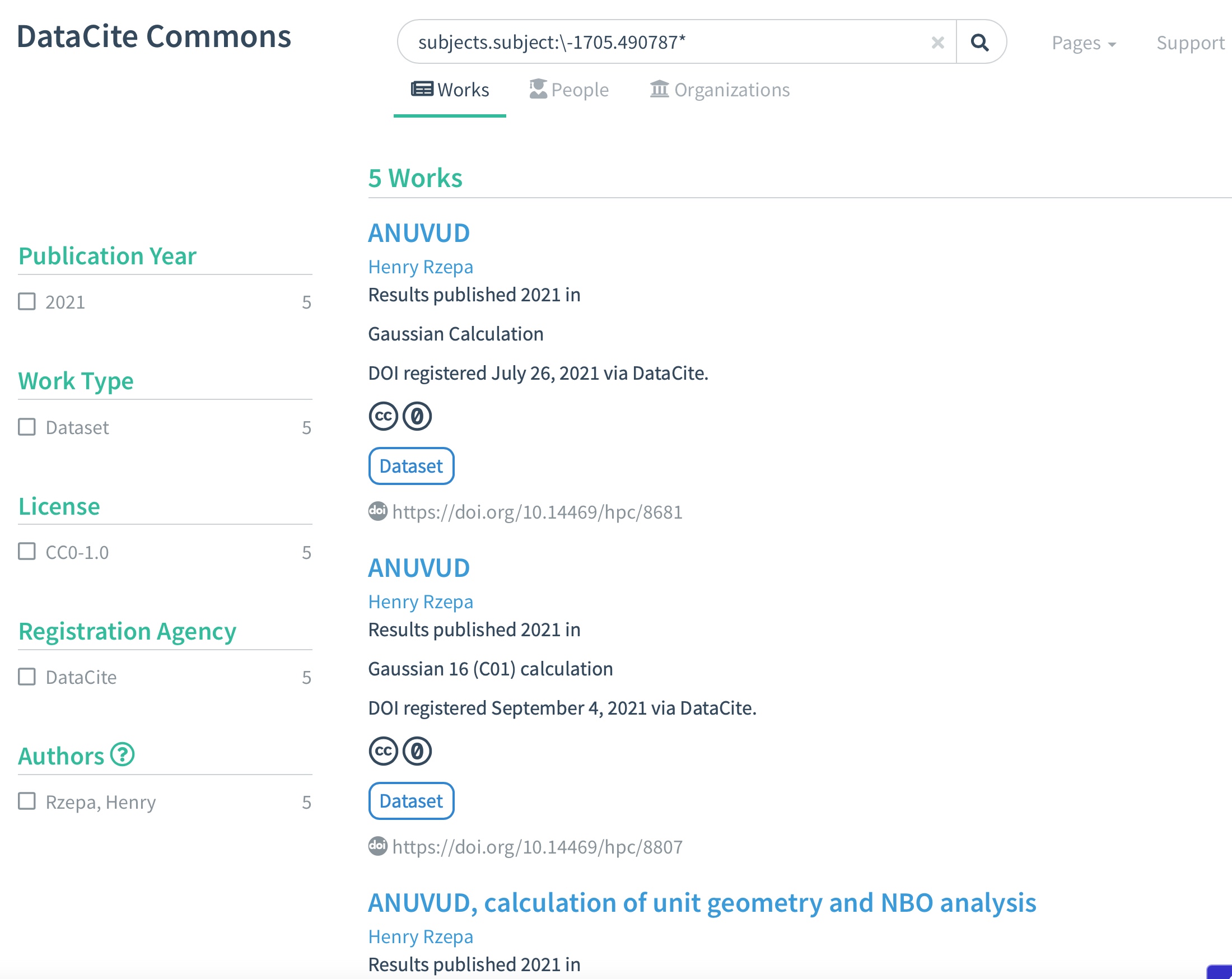
In the previous blog post, I looked at the metadata records registered with DataCite for some chemical computational modelling files as published in three different repositories. Here I take it one stage further, by looking at how searches of the DataCite metadata store for three particular values of the metadata associated with this dataset compare.

The number of repositories which accept research data across a wide spectrum of disciplines is on the up. Here I report the results of conducting an experiment in which chemical modelling data was deposited in six such repositories and comparing the richness of the metadata describing the essential properties of the six depositions.
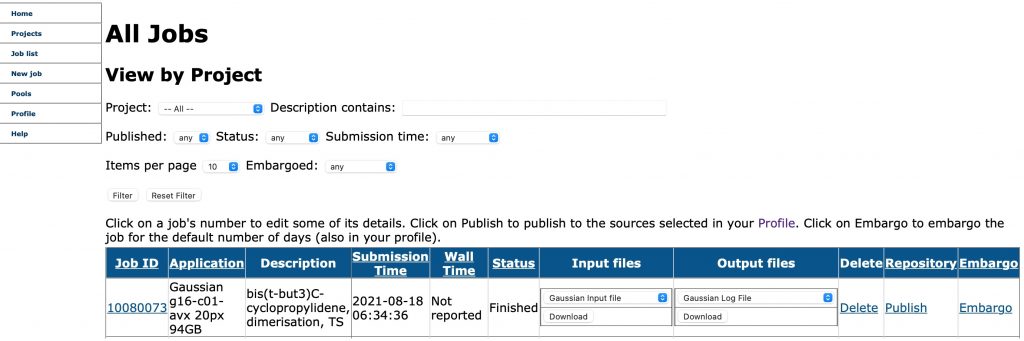
You might have noticed if you have read any of my posts here is that many of them have been accompanied since 2006 by supporting calculations, normally based on density functional theory (DFT) and these calculations are accompanied by a persistent identifier pointer‡ to a data repository publication.
An earlier post investigated large anomeric effects involving two oxygen atoms attached to a common carbon atom.
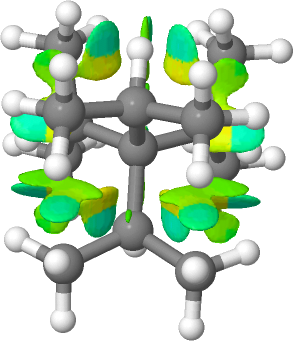
The homologous hydrocarbon series R4C is known for R=Me as neopentane and for R=Et as 3,3-diethylpentane. The next homologue, R=iPr bis(3,3-isopropyl)-2,4-dimethylpentane is also a known molecule for which a crystal structure has been reported (DOI: https://doi.org/10.5517/cc4wvnh). The final member of the series, R= tbutyl is unknown.
Whilst I was discussing the future of scientific publication in the last post, a debate was happening behind the scenes regarding the small molecule cyclopropenylidene. This is the smallest known molecule displaying π-aromaticity, but its high reactivity means that it is unlikely to be isolated in the condensed phase.
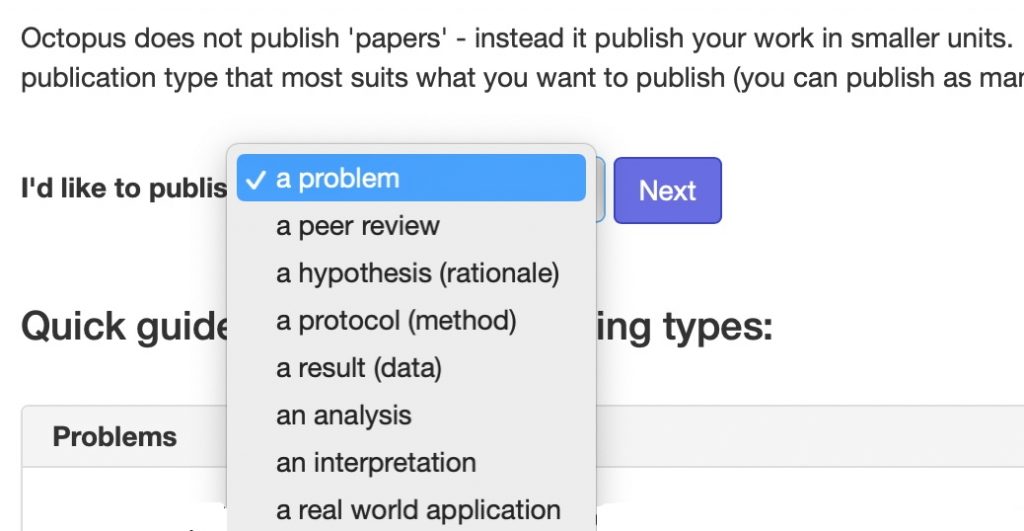
In 2011, I suggested that the standard monolith that is the conventional scientific article could be broken down into two separate, but interlinked components, being the story or narrative of the article and the data on which the story is based.
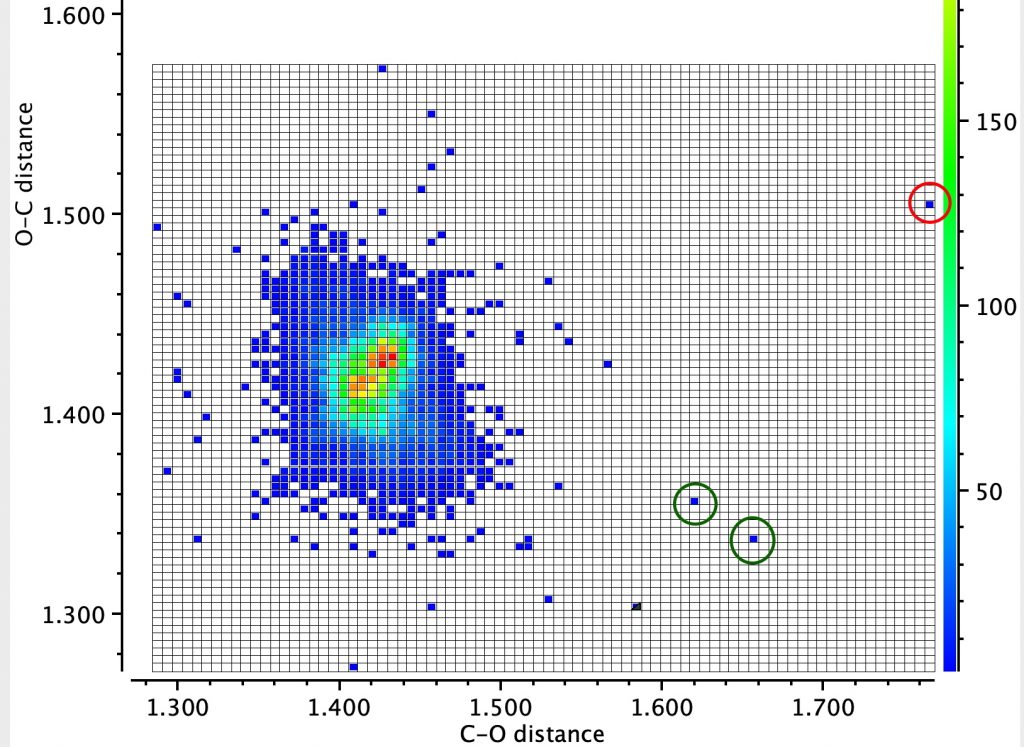
In the earlier post on the topic of anomeric effects, I identified a number of outliers associated with large differences in the lengths of two carbon-oxygen bonds sharing a common carbon atom.