In the previous post, I showed that carbon can act as a hydrogen bond acceptor (of a proton) to form strong hydrogen bond complexes. Which brings me to a conceptual connection: can singlet dicarbon form such a hydrogen bond?
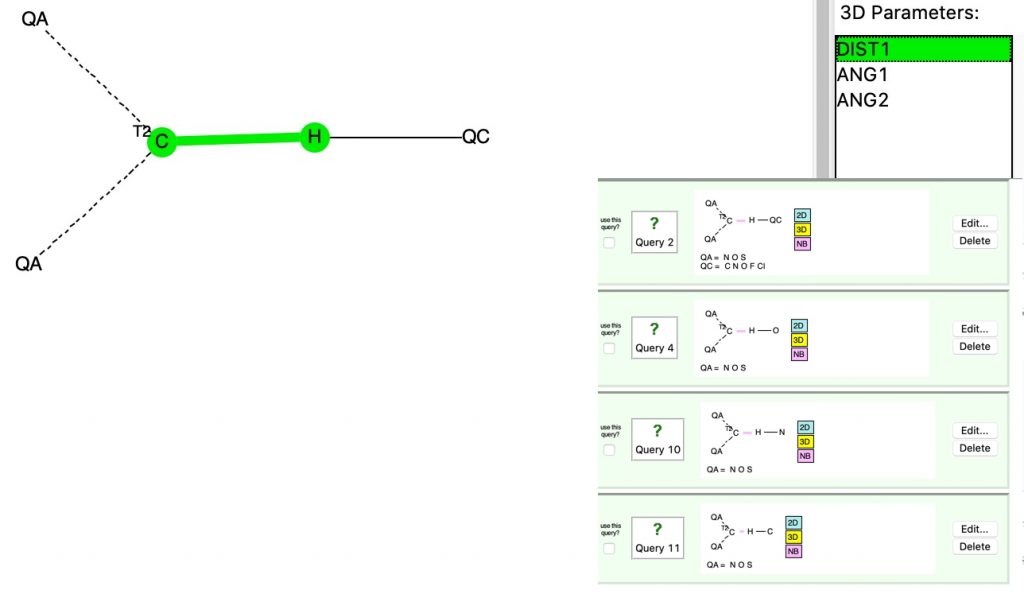
A hydrogen bond donor is considered as an electronegative element carrying a hydrogen that is accepted by an atom carrying a lone pair of electrons, as in X:…H-Y where X: is the acceptor and H-Y the donor.
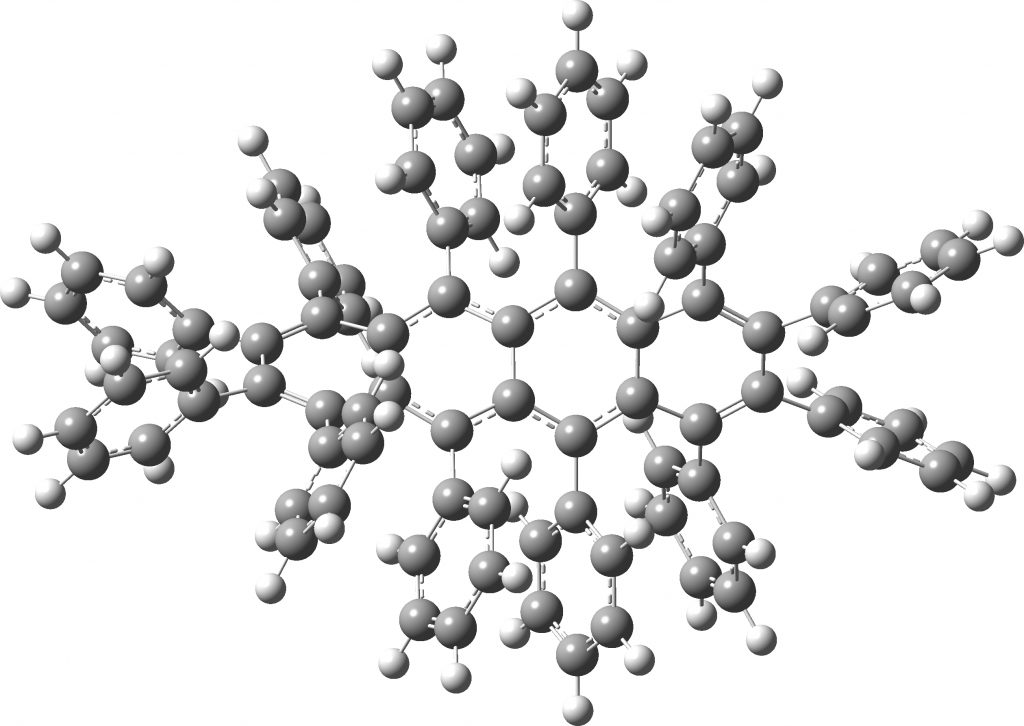
All of the molecules in this year’s C&EN list are fascinating in their very different ways. Here I take a look at the twisty tetracene (dodecaphenyltetracene) which is indeed very very twisty.
My momentum of describing early attempts to use optical rotation to correlate absolute configuration of small molecules such as glyceraldehyde and lactic acid with their optical rotations has carried me to L-Malic acid (below labelled as (S)-Malic acid).
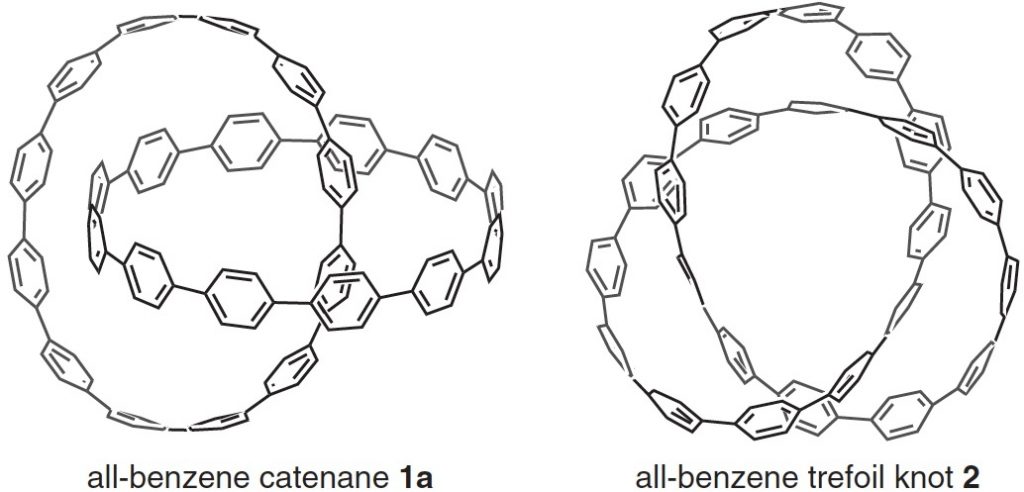
Here is another molecule of the year, on a topic close to my heart, the catenane systems 1 and the trefoil knot 2 Such topology is closely inter-twinned with three dimensions (literally) and I always find that the flat pages of a journal are simply insufficient to do them justice.
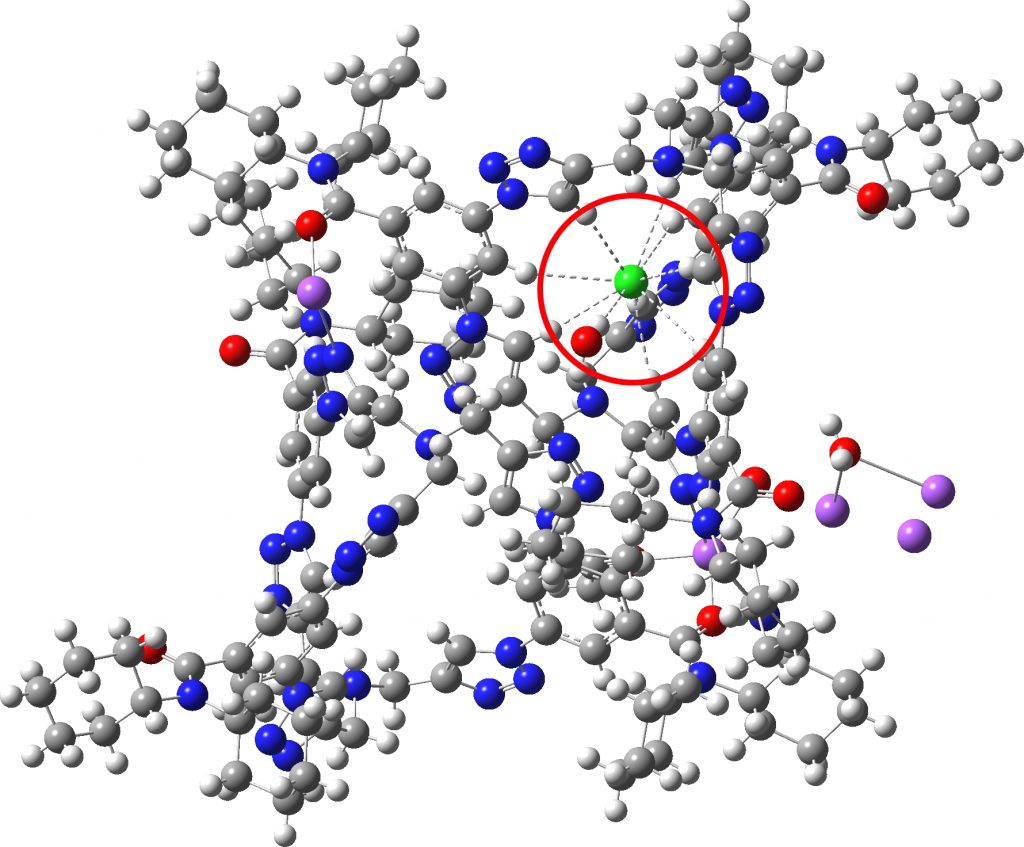
Each year, C&E News runs a poll for their “Molecule of the year“. I occasionally comment with some aspect of one of the molecules that catches my eye (I have already written about cyclo[18]carbon, another in the list). Here, it is the Incredible chloride cage, a cryptand-like container with an attomolar (1017 M-1) affinity for […]
I have been discussing some historical aspects of the absolute configuration of molecules and how it was connected to their optical rotations.
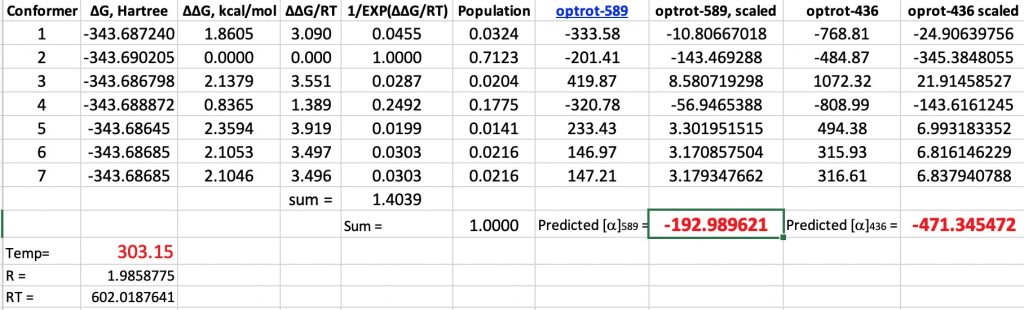
In this series of posts on optical rotations, I firstly noted Kirkwood’s 1937 attempt to correlate the optical rotation of butan-2-ol with its absolute configuration.
Text books often show the following diagram, famously consolidated over many years by Emil Fischer from 1891 onwards. At the top sits D-(+)-glyceraldehyde, to which all the monosaccharides below are connected by painstaking chemical transformations.
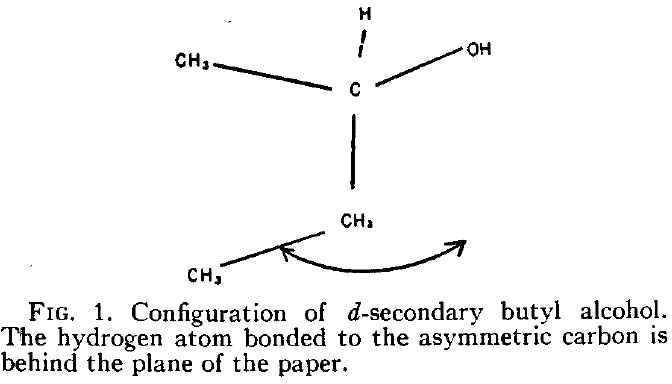
Some areas of science progressed via very famous predictions that were subsequently verified by experiments. Think of Einstein and gravitational waves or of Dirac and the positron. There are fewer well-known examples in chemistry; perhaps Watson and Crick’s prediction of the structure of DNA, albeit based on the interpretation of an existing experimental result.
In the previous post, I discussed the structure of the free base form of tetrodotoxin, often represented as originally suggested by Woodward below in an ionic form: