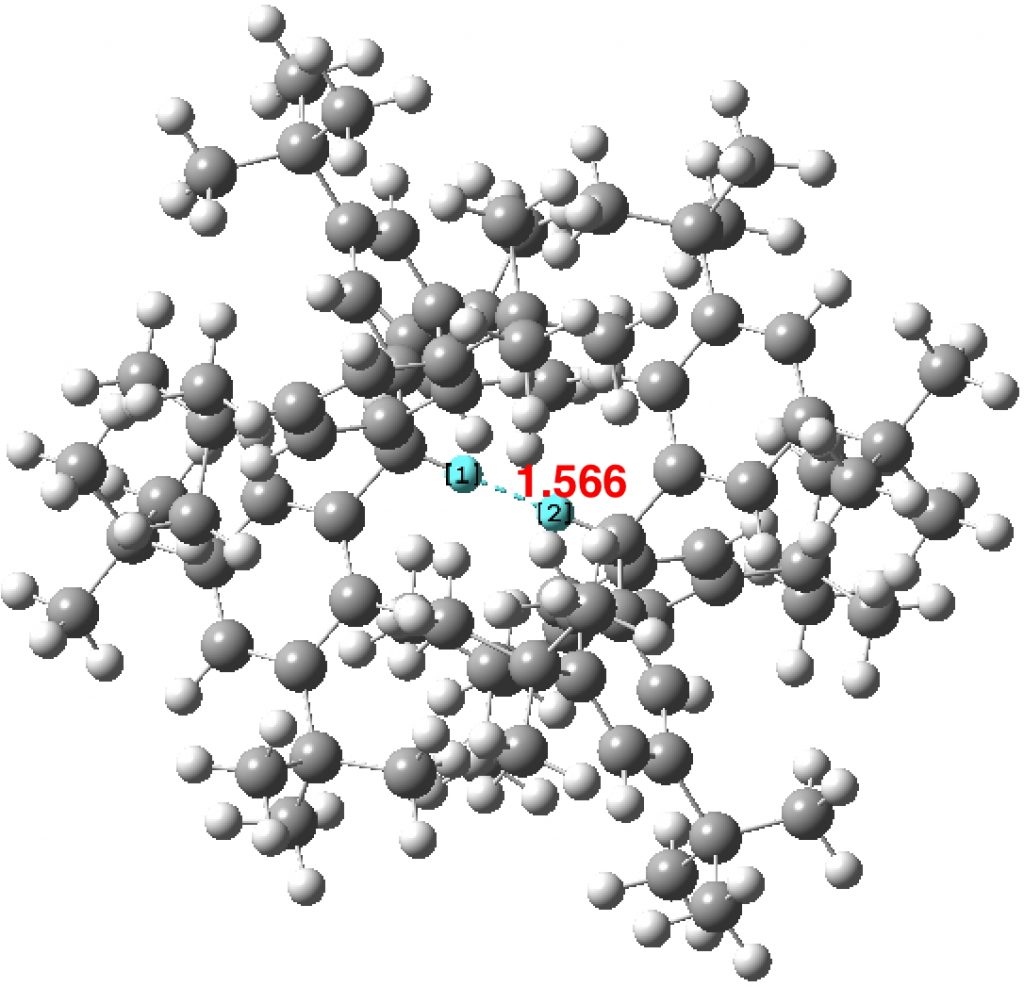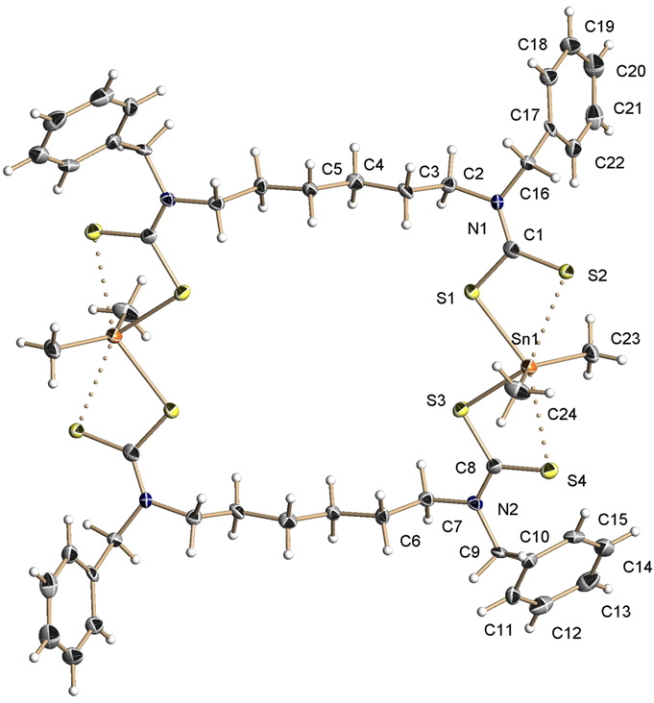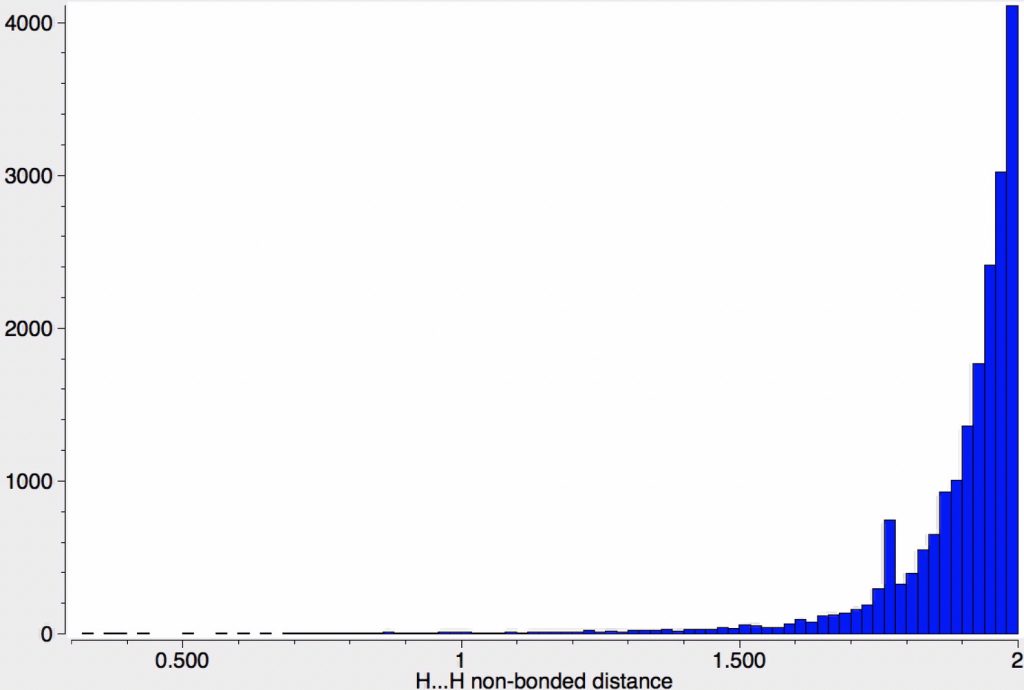
In the previous post, I noted the crystallographic detection of an unusually short non-bonded H…H contact of ~1.5Å, some 0.9Å shorter than twice the van der Waals radius of hydrogen (1.2Å, although some sources quote 1.1Å which would make the contraction ~0.7Å). This was attributed to dispersion attractions accumulating in the rest of the molecule.