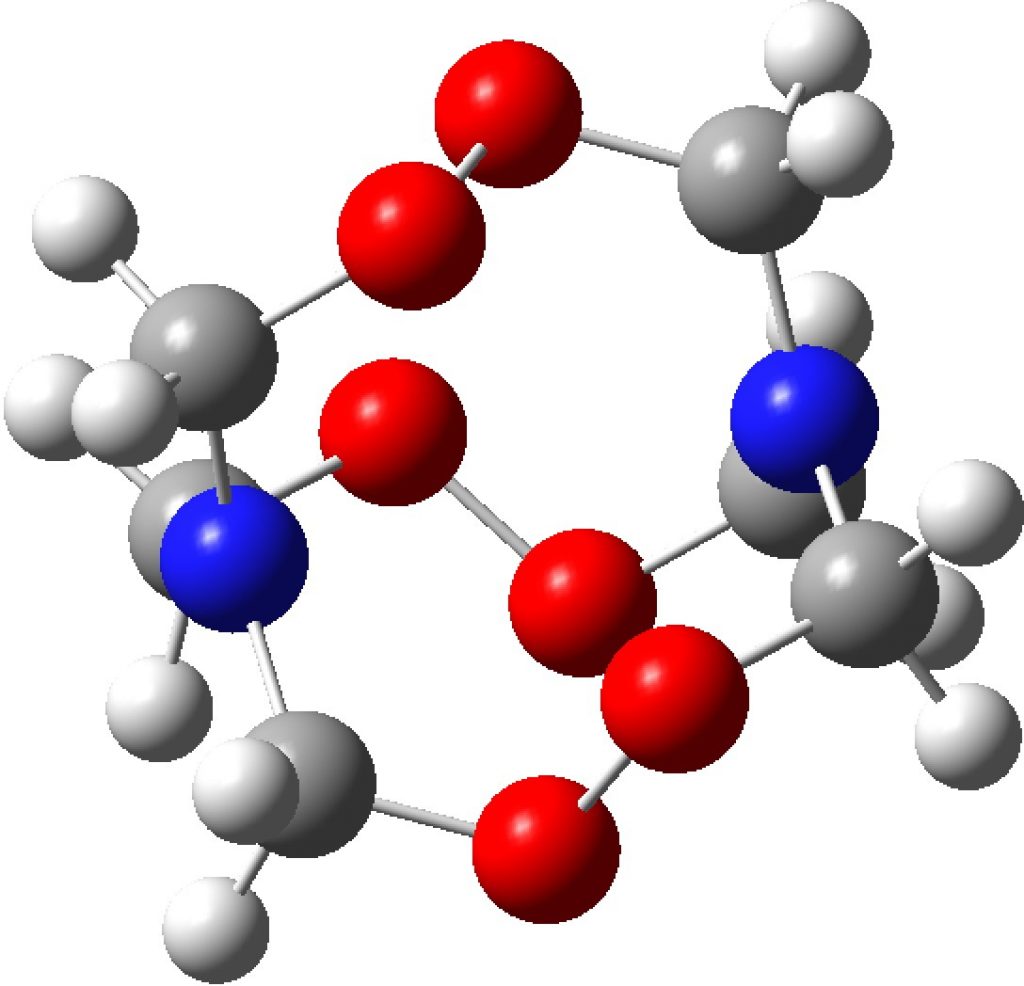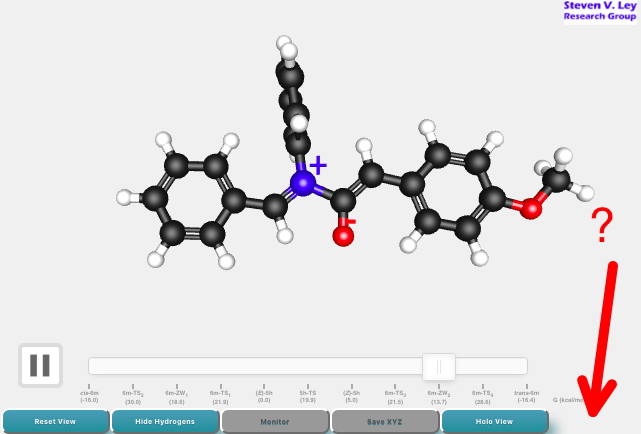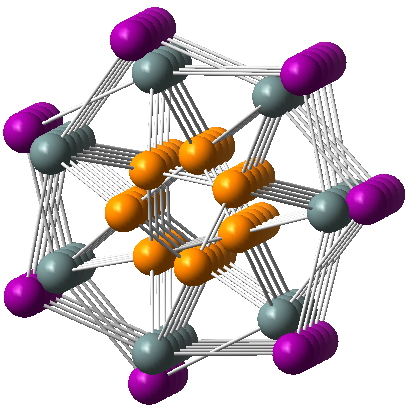
After sixty years of searching, the first non-templated double helical carbon-free inorganic molecular structure has been reported. That is so neat that I thought to load the 3D coordinates here for you to interact with and then to explore the prospect of using these coordinates to add some value with e.g. some chiroptical calculations.