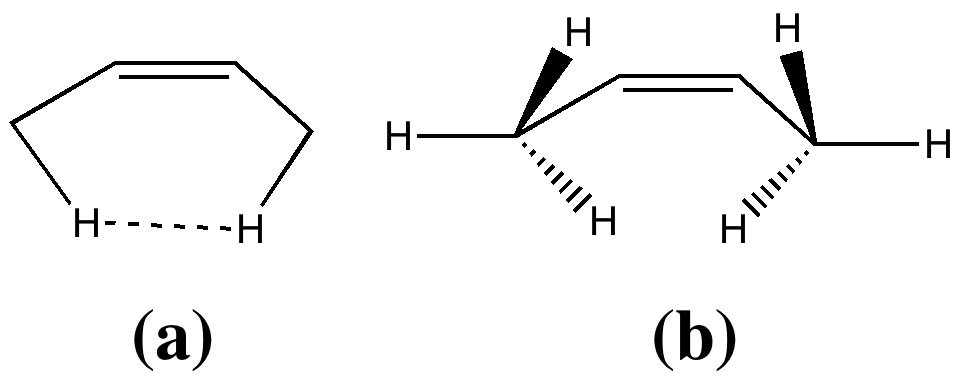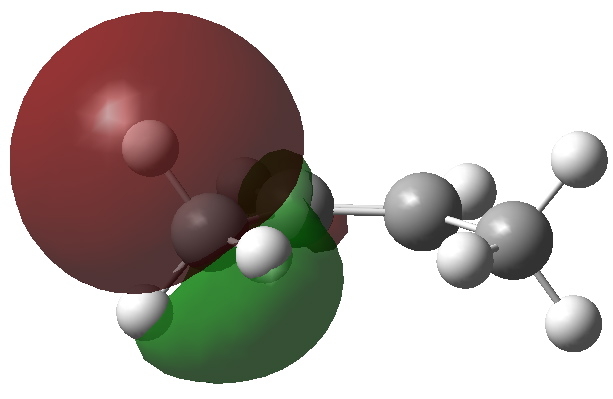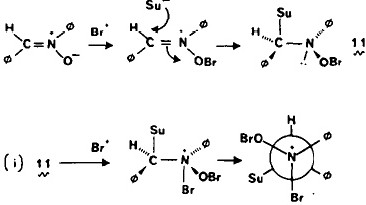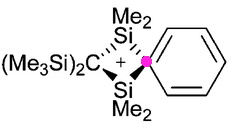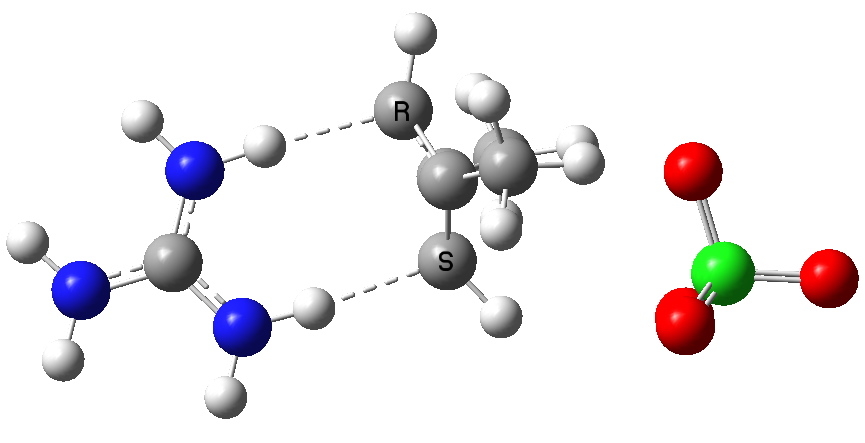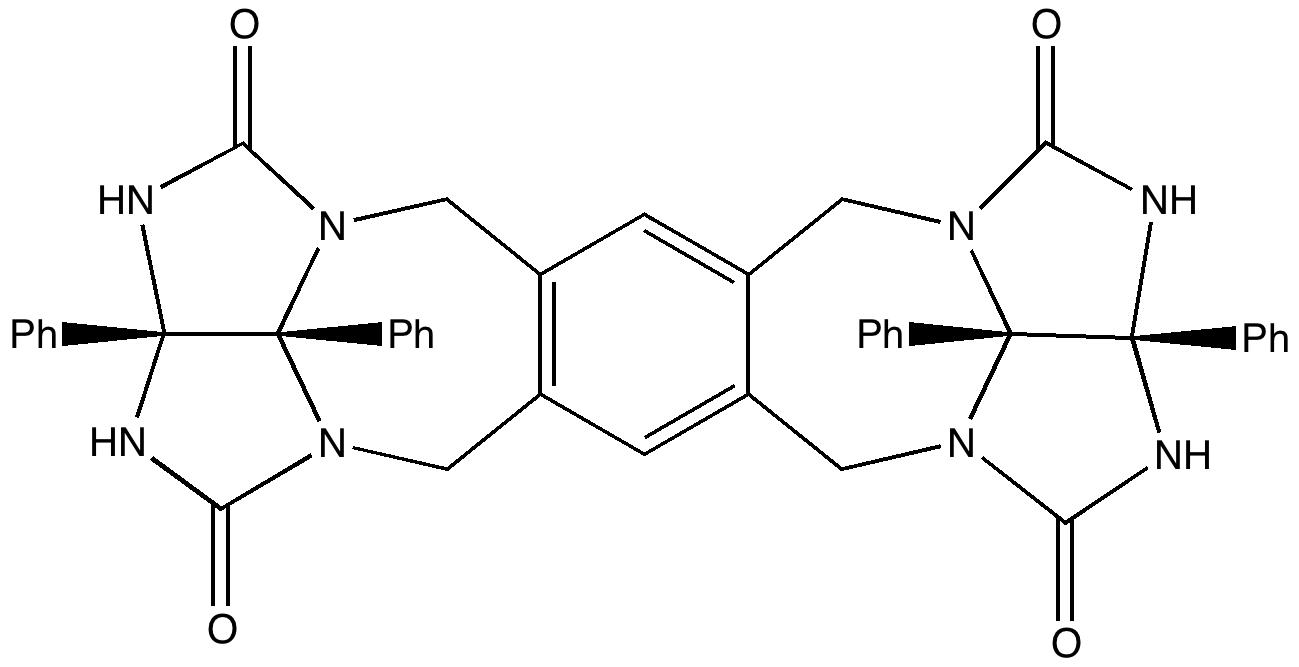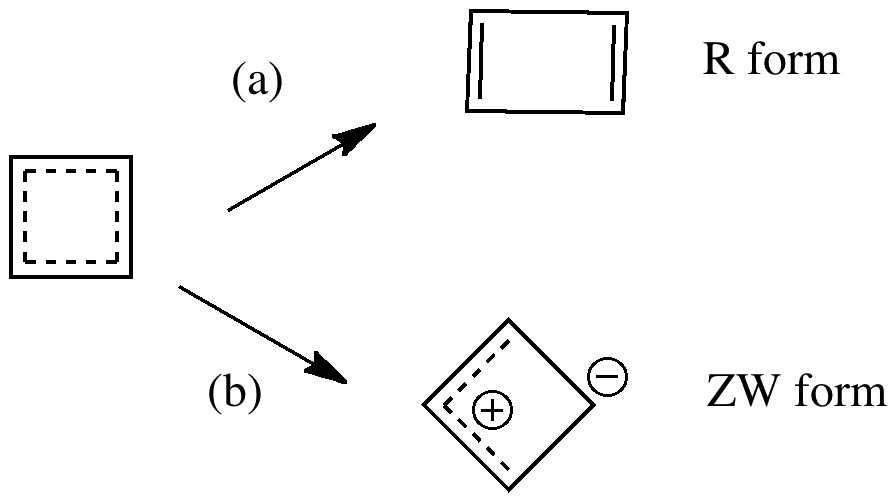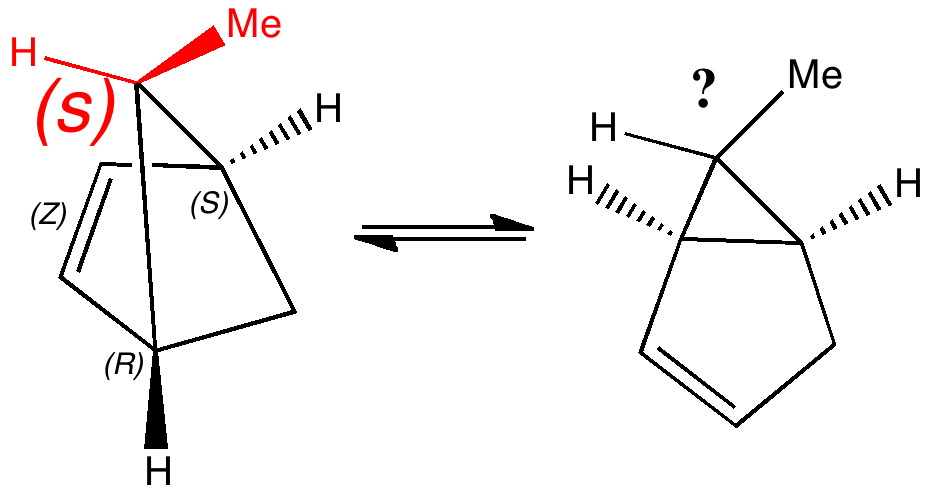
Most representational chemistry generated on a computer requires the viewer to achieve a remarkably subtle transformation in their mind from two to three dimensions (we are not quite yet in the era of the 3D iPad!). The Cahn-Ingold-Prelog convention was a masterwork (which won the Nobel prize). It is shown in action for the molecule […]