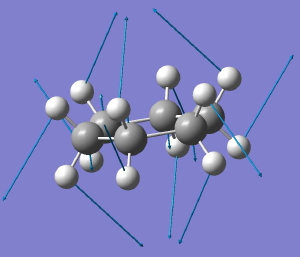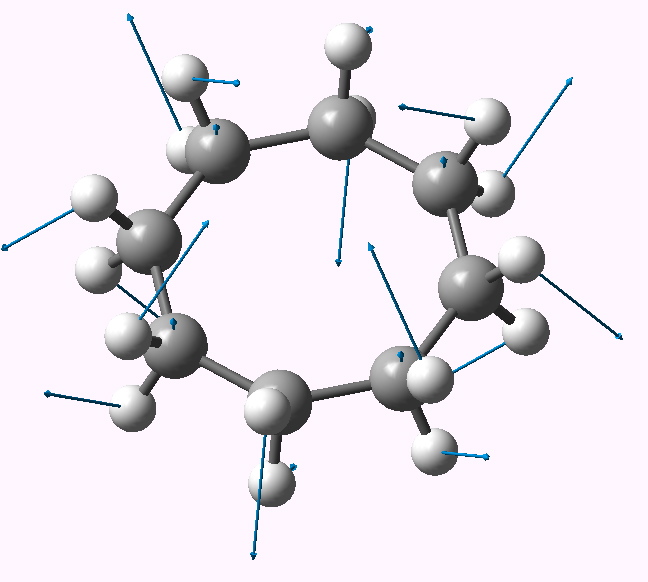
In the previous post, I suggested that inspecting the imaginary modes of planar cyclohexane might be a fruitful and systematic way in which at least parts of the conformational surface of this ring might be probed. Here, the same process is conducted for cyclo-octane.