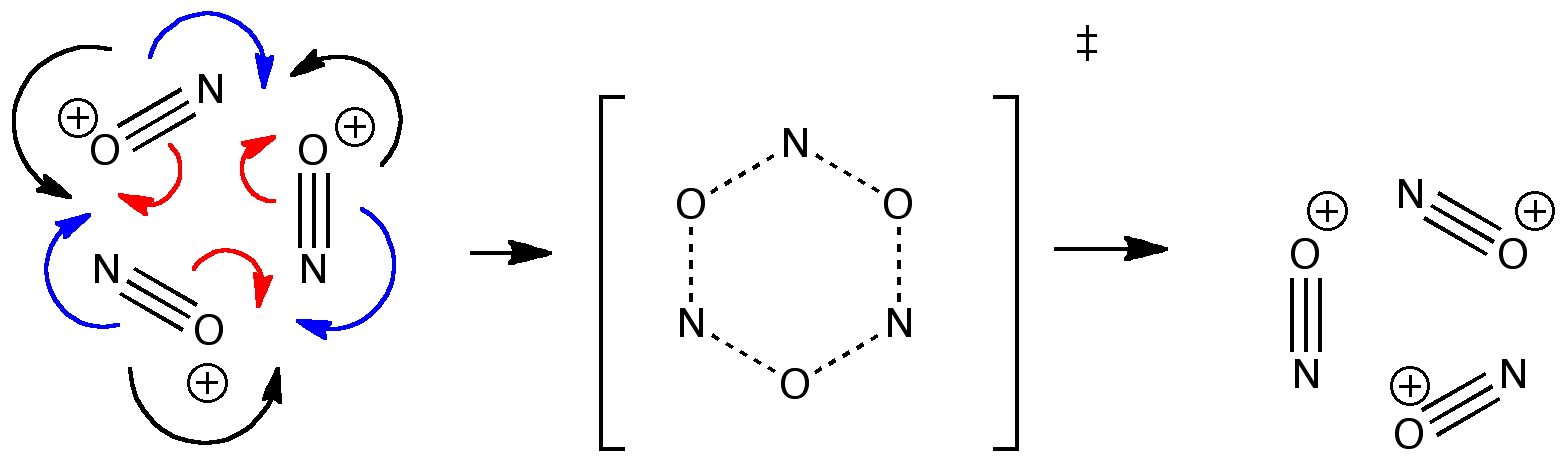
In my first post on this theme, an ELF (Electron localization function) analysis of the bonding in the molecule HO-S≡C-H (DOI: 10.1002/anie.200903969) was presented. This analysis identified a lone pair of electrons localized on the carbon (integrating in fact to almost exactly 2.0) in addition to electrons in the CC region.